Question
Question: The number of the cases in which the rate of $S_N2$ reaction is greater in the second compound as co...
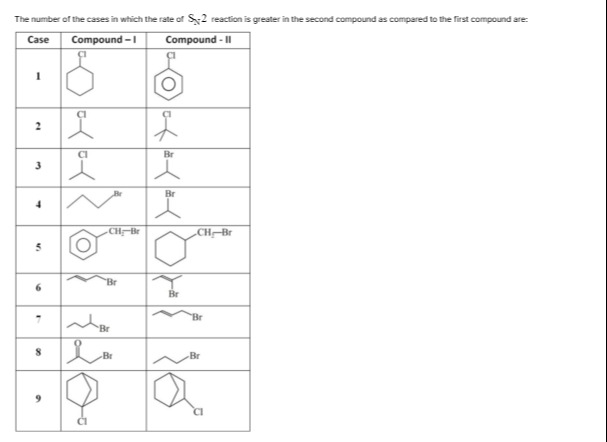
The number of the cases in which the rate of SN2 reaction is greater in the second compound as compared to the first compound are:
| Case | Compound - I | Compound - II |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 7 | ||
| 8 | ||
| 9 |
3
Solution
To determine the cases where the rate of SN2 reaction is greater in the second compound (Compound II) compared to the first compound (Compound I), we need to analyze the factors affecting SN2 reaction rates:
- Steric Hindrance: Lower steric hindrance at the carbon bearing the leaving group leads to a faster SN2 reaction. The general order of reactivity is methyl > primary (1°) > secondary (2°) > tertiary (3°). Tertiary halides, vinyl halides, aryl halides, and bridgehead halides are generally unreactive in SN2 reactions.
- Leaving Group Ability: A better leaving group leads to a faster SN2 reaction. The order of common leaving groups is I⁻ > Br⁻ > Cl⁻ > F⁻.
- Electronic Effects: Electron-withdrawing groups can increase the electrophilicity of the carbon undergoing attack, potentially enhancing SN2 reactivity.
Let's analyze each case:
Case 1:
- Compound I: Cyclohexyl chloride (2° alkyl halide) - SMILES:
C1CCCCC1Cl - Compound II: Chlorobenzene (Aryl halide) - SMILES:
Clc1ccccc1
Aryl halides are unreactive towards SN2 due to the partial double bond character of the C-Cl bond and steric/geometric constraints for backside attack. 2° alkyl halides undergo SN2. Therefore, Rate(I) > Rate(II). (II is NOT faster)
Case 2:
- Compound I: Isopropyl chloride (2° alkyl halide) - SMILES:
CC(C)Cl - Compound II: tert-butyl chloride (3° alkyl halide) - SMILES:
CC(C)(C)Cl
3° alkyl halides are essentially unreactive towards SN2 due to high steric hindrance. 2° alkyl halides react via SN2. Therefore, Rate(I) > Rate(II). (II is NOT faster)
Case 3:
- Compound I: Isopropyl chloride (2° alkyl halide, leaving group Cl) - SMILES:
CC(C)Cl - Compound II: Isopropyl bromide (2° alkyl halide, leaving group Br) - SMILES:
CC(C)Br
Both are 2° alkyl halides, so steric hindrance is comparable. Br⁻ is a better leaving group than Cl⁻. Therefore, Rate(II) > Rate(I). (II IS faster)
Case 4:
- Compound I: 1-Bromopropane (1° alkyl halide) - SMILES:
CCCBr - Compound II: Isopropyl bromide (2° alkyl halide) - SMILES:
CC(C)Br
1° alkyl halides are less sterically hindered than 2° alkyl halides, leading to faster SN2. Therefore, Rate(I) > Rate(II). (II is NOT faster)
Case 5:
- Compound I: Benzyl bromide (1° alkyl halide) - SMILES:
BrCC1=CC=CC=C1 - Compound II: Cyclohexylmethyl bromide (1° alkyl halide) - SMILES:
BrCC1CCCCC1
Both are primary alkyl halides. The cyclohexyl group is generally considered bulkier than the planar phenyl group. Less steric hindrance at the reaction center leads to faster SN2. Therefore, Rate(I) > Rate(II). (II is NOT faster)
Case 6:
- Compound I: Allyl bromide (1° alkyl halide) - SMILES:
C=CCBr - Compound II: 2-Bromopropene (Vinyl halide) - SMILES:
CC(Br)=C
Vinyl halides are unreactive towards SN2. Allyl halides (primary) are reactive. Therefore, Rate(I) > Rate(II). (II is NOT faster)
Case 7:
- Compound I: 2-Bromobutane (2° alkyl halide) - SMILES:
CCC(C)Br - Compound II: 1-Bromobut-2-ene (Allylic 1° alkyl halide) - SMILES:
CC=CCBr
Allylic primary halides (Compound II) are less sterically hindered than secondary alkyl halides (Compound I). Therefore, Rate(II) > Rate(I). (II IS faster)
Case 8:
- Compound I: 2-Bromo-1-phenylethanone (α-bromo ketone, 1° alkyl halide) - SMILES:
O=C(CBr)c1ccccc1 - Compound II: 1-Bromopropane (1° alkyl halide) - SMILES:
CCCBr
Both are primary alkyl halides. The electron-withdrawing carbonyl group in Compound I makes the α-carbon more electrophilic and stabilizes the SN2 transition state, significantly enhancing SN2 reactivity compared to a simple primary alkyl halide. Therefore, Rate(I) > Rate(II). (II is NOT faster)
Case 9:
- Compound I: 1-Chlorobicyclo[2.2.1]heptane (Bridgehead halide) - SMILES:
ClC1C2CCC1C2 - Compound II: 2-Chlorobicyclo[2.2.1]heptane (2° alkyl halide) - SMILES:
ClC1C2CCC(C1)C2
Bridgehead halides (Compound I) are unreactive towards SN2 because backside attack is geometrically impossible. 2° alkyl halides (Compound II) undergo SN2. Therefore, Rate(II) > Rate(I). (II IS faster)
The cases where the rate of SN2 reaction is greater in the second compound as compared to the first compound are cases 3, 7, and 9. The total number of such cases is 3.
