Question
Question: The number of substrates which neither show $S_N1$ nor $S_N2$....
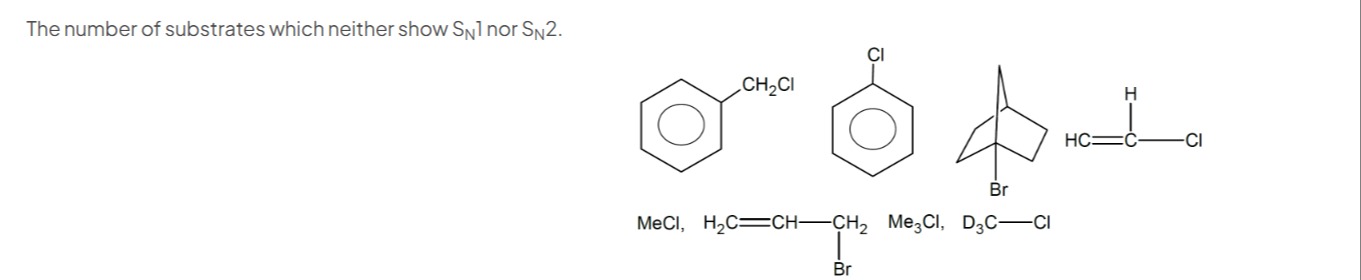
The number of substrates which neither show SN1 nor SN2.
3
Solution
To determine which substrates neither show SN1 nor SN2 reactions, we need to analyze the structural features of each compound and their implications for nucleophilic substitution mechanisms.
General Principles:
- SN1 Reaction: Favored by stable carbocation intermediates. Reactivity order: 3° > 2° > 1° > methyl. Vinylic, aryl, and bridgehead halides generally do not undergo SN1 due to unstable carbocation formation or inability to achieve planar geometry.
- SN2 Reaction: Favored by less steric hindrance around the carbon bearing the leaving group. Reactivity order: methyl > 1° > 2° > 3°. Vinylic, aryl, and bridgehead halides generally do not undergo SN2 due to partial double bond character of the C-X bond, less electrophilic sp2 carbon, or steric hindrance preventing backside attack.
Let's analyze each substrate:
-
Benzyl chloride (Ph-CH₂Cl)
- SN1: Forms a resonance-stabilized benzylic carbocation (Ph-CH₂⁺). Thus, it undergoes SN1.
- SN2: It is a primary halide with relatively low steric hindrance. Thus, it undergoes SN2.
- Conclusion: Shows both SN1 and SN2.
-
Chlorobenzene (Ph-Cl)
- SN1: Forms a phenyl carbocation (Ph⁺), which is highly unstable (positive charge on an sp2 carbon in a rigid ring). Thus, it does not undergo SN1.
- SN2: The C-Cl bond has partial double bond character due to resonance with the benzene ring, making it stronger and shorter. The sp2 carbon is also less electrophilic, and steric hindrance from the ortho hydrogens impedes backside attack. Thus, it does not undergo SN2 under normal conditions.
- Conclusion: Neither SN1 nor SN2. (Count = 1)
-
1-Bromo-bicyclo[2.2.1]heptane (Norbornyl bromide)
- SN1: Forms a bridgehead carbocation. This carbocation cannot achieve the required planar geometry due to the rigid bicyclic structure (violates Bredt's Rule), making it highly unstable. Thus, it does not undergo SN1.
- SN2: Backside attack by a nucleophile is sterically impossible due to the rigid cage-like structure. Thus, it does not undergo SN2.
- Conclusion: Neither SN1 nor SN2. (Count = 2)
-
Vinyl chloride (H₂C=CH-Cl)
- SN1: Forms a vinylic carbocation (H₂C=CH⁺), which is highly unstable (positive charge on an sp2 carbon). Thus, it does not undergo SN1.
- SN2: The carbon bearing the halogen is sp2 hybridized, making the C-Cl bond stronger (partial double bond character) and less susceptible to nucleophilic attack. Thus, it does not undergo SN2 under normal conditions.
- Conclusion: Neither SN1 nor SN2. (Count = 3)
-
Methyl chloride (MeCl or CH₃Cl)
- SN1: Forms a highly unstable methyl carbocation (CH₃⁺). Thus, it does not undergo SN1 (or is extremely slow).
- SN2: It is a methyl halide, which is the least sterically hindered, making it highly reactive towards SN2. Thus, it undergoes SN2.
- Conclusion: Shows SN2, not SN1.
-
H₂C=CH-CH₂-CH₂Br (1-bromobut-3-ene) (Assuming this interpretation of the ambiguous drawing)
- SN1: Forms a primary carbocation (H₂C=CH-CH₂-CH₂⁺), which is unstable. Thus, it does not undergo SN1 (or is extremely slow).
- SN2: It is a primary alkyl halide with low steric hindrance. Thus, it readily undergoes SN2.
- Conclusion: Shows SN2, not SN1.
(Note: If the structure was intended to be allyl bromide (H₂C=CH-CH₂Br), it would show both SN1 and SN2 due to resonance stabilization of the allylic carbocation and primary nature for SN2.)
-
Trimethylchloromethane (Me₃CCl or (CH₃)₃CCl)
- SN1: Forms a highly stable tertiary carbocation ((CH₃)₃C⁺). Thus, it readily undergoes SN1.
- SN2: It is a tertiary halide, highly sterically hindered, preventing backside attack. Thus, it does not undergo SN2.
- Conclusion: Shows SN1, not SN2.
-
Trideuteromethyl chloride (D₃CCl)
- SN1: Chemically similar to methyl chloride, forms a highly unstable methyl carbocation (CD₃⁺). Thus, it does not undergo SN1 (or is extremely slow).
- SN2: Chemically similar to methyl chloride, it is a methyl halide, least sterically hindered. Thus, it readily undergoes SN2.
- Conclusion: Shows SN2, not SN1.
Summary of "Neither SN1 nor SN2" substrates:
- Chlorobenzene
- 1-Bromo-bicyclo[2.2.1]heptane
- Vinyl chloride
There are 3 such substrates.
