Question
Question: The number of structurally isomeric esters with molecular formula \[{{\text{C}}_{\text{5}}}{{\text{H...
The number of structurally isomeric esters with molecular formula C5H10O2 are
A) 3
B) 5
C) 6
D) 8
Solution
Using the given molecular formula of ester determines the degrees of unsaturation. Ester is a carboxylic derivative of acid. The general formula of the ester is RCOOR’. Isomers are the compound having the same molecular formula but a different structural arrangement.
Complete solution:
We have to determine the structural isomers of ester having molecular formulaC5H10O2.
To determine the structures of isomers we have to determine the index of hydrogen deficiency (IHD) which is also known as units of unsaturation using the following formula.
IHD = 0.5×[2c + 2 - h - x + n]
Where,
c = number of carbon atoms = 5
h = number of hydrogen atoms = 10
x = number of halogen atoms = 0
n = number of nitrogen atoms = 0
IHD = 0.5×[2×5 + 2 - 10 - 0 + 0]
IHD = 1
This indicates that ester having molecular formulaC5H10O2 contains one double bond of carbonyl carbon.
The general structure of ester is
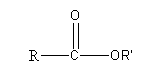
Here, R and R’ are two different alkyl groups. By using a different type of alkyl groups we can draw the different isomers of ester having a formulaC5H10O2 as follows:
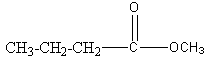
Structure I
Now we will keep COOCH3 the group fix and will write the other possible isomers.
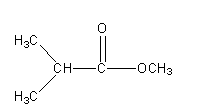
Structure II
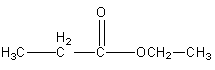
Now we will write the isomers having a different OR’ group.
Structure III
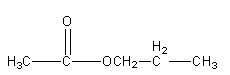
Structure IV
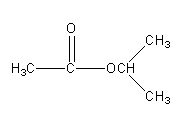
So, there are 5 isomeric esters possible having molecular formula C5H10O2 .
Hence, the option (B) is the correct answer to the question.
Note: Ester is one of the organic functional groups. The molecular formula of all isomers is the same only the arrangement of bonding atoms is different. Ester contains two types of alkyl groups. One alkyl group bonded to carbonyl carbon and one alkyl bonded to the oxygen atom of the carboxylate group. By varying the alkyl groups we can draw all possible isomers of ester.
