Question
Question: The number of structural isomers possible with the formula \({C_4}{H_9}Cl\) are: A. 5 B. 4 C. ...
The number of structural isomers possible with the formula C4H9Cl are:
A. 5
B. 4
C. 3
D. 2
Solution
The structural isomers are defined as the organic compounds which have similar molecular formula but differ in their structural arrangement. The structural isomers are possible in hydrocarbons where more than two carbon atoms are present In C4H9Cl, four carbon atoms are present.
Complete step by step answer:
The isomers are defined as the molecules which possess the same molecular formulas but differ in the arrangement of atoms and groups. Isomers are divided into conformational isomers, structural isomers, stereoisomers, geometric isomers, optical isomers.
The structural isomers are defined as the organic compounds which have similar molecular formula but differ in their structural arrangement.
In haloalkanes, the number of carbon atoms, hydrogen atom and halogen atom are the same but the arrangement of carbon atoms, hydrogen atoms and halogen atoms are different.
The chemical formula given is C4H9Cl, where four carbon atoms are present, nine hydrogen atoms are present and one chlorine atom is present.
To draw the structural isomers of C4H9Cl, first draw a straight chain butane hydrocarbon and replace one hydrogen with the chlorine atom to form 1-chloro-2-methylpropane and 2-chloro-2methyl methylpropane. Secondly draw three
The structural isomers of C4H9Cl are shown below.
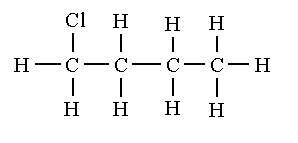
1-chlorobutane
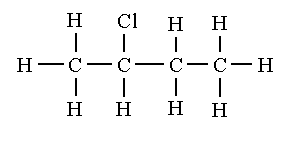
2-chlorobutane
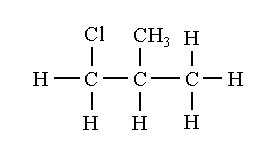
1-chloro-2-methylpropane
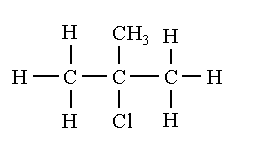
So, the correct answer is “Option B”.
Note:
The structural isomers are also called constitutional isomers. The structural isomers of alkyl halide are all different compounds therefore, they all possess different physical and chemical properties.
