Question
Question: The number of species from the following that are involved in $sp^3d^2$ hybridization is $[Co(NH_3)_...
The number of species from the following that are involved in sp3d2 hybridization is [Co(NH3)6]3+, SF6, [CrF6]3−, [CoF6]3−, [Mn(CN)6]3− and [MnCl6]3−.
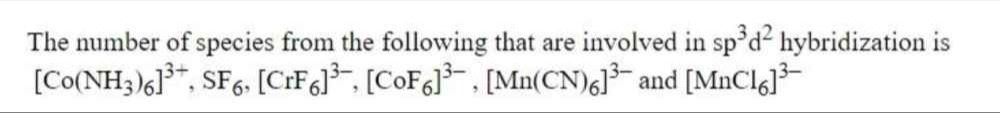
Answer
3
Explanation
Solution
For an octahedral complex, the inner orbital (d²sp³) hybridization is used when the metal ion has low spin configuration (often with strong‐field ligands) because two 3d orbitals remain vacant, while the outer orbital (sp³d²) hybridization is used in high spin complexes (with weak‐field ligands) when all the 3d orbitals are occupied or insufficient vacancies exist.
- [Co(NH3)6]3+: Co(III) with NH3 (a moderately strong field ligand) is low spin → uses d2sp3.
- SF6: Sulfur uses 3s, 3p, and 3d orbitals → sp3d2 hybridization.
- [CrF6]3−: Cr(III) (d3) has three electrons occupying t2g, leaving two inner d‐orbitals free → d2sp3 hybridization.
- [CoF6]3−: Co(III) with F⁻ (a weak field ligand) is forced high spin (d6 high spin) and does not have two vacant 3d orbitals → uses sp3d2 hybridization.
- [Mn(CN)6]3−: Mn(III) with CN⁻ (a strong field ligand) is low spin (d4 low spin) → uses d2sp3 hybridization.
- [MnCl6]3−: Mn(III) with Cl⁻ (a weak field ligand) is high spin (d4 high spin) → uses sp3d2 hybridization.
Thus, the species with sp3d2 hybridization are:
- SF6
- [CoF6]3−
- [MnCl6]3−
