Question
Question: The number of sigma (\(\sigma \)) and pi (\(\pi \)) bonds present in 2-Formyl benzoic acid are: (a...
The number of sigma (σ) and pi (π) bonds present in 2-Formyl benzoic acid are:
(a)- 15σ, 4π
(b)- 15σ, 5π
(c)- 17σ, 6π
(d)- 17σ, 5π
Solution
The sigma bond is shown as σ−bond and pi bond are shown as π−bond, if all the bonds in the compound are single then there are only sigma bonds, and pi bonds are present in the double and the triple bonds. 2-Formyl benzoic acid is an aromatic compound in which there is a benzene ring and there is a carboxylic acid present on the benzene ring, and at the second carbon atom, an aldehyde group is present.
Complete answer:
If the compounds are covalent, it means that there are covalent bonds in them, and there are two types of covalent bonds, i.e., sigma bond shown as σ−bond and pi bond shown as π−bond.
By counting the number of bonds, we can calculate the number of sigma and pi bonds. Sigma and pi bonds are based on the type of bond present in the molecule, i.e., a single bond, double bond, and triple bond. Therefore, in single bonds, only a sigma bond is present, in the double bond one is sigma and one is pi, and in the triple bond one is a sigma bond and two are pi bonds.
2-Formyl benzoic acid is an aromatic compound in which there is a benzene ring and there is a carboxylic acid present on the benzene ring, and at the second carbon atom, an aldehyde group is present. So, in this compound, there is no triple bond and there is a total of 5 double bonds. The total number of bonds is 22. The structure of the compound is given below:
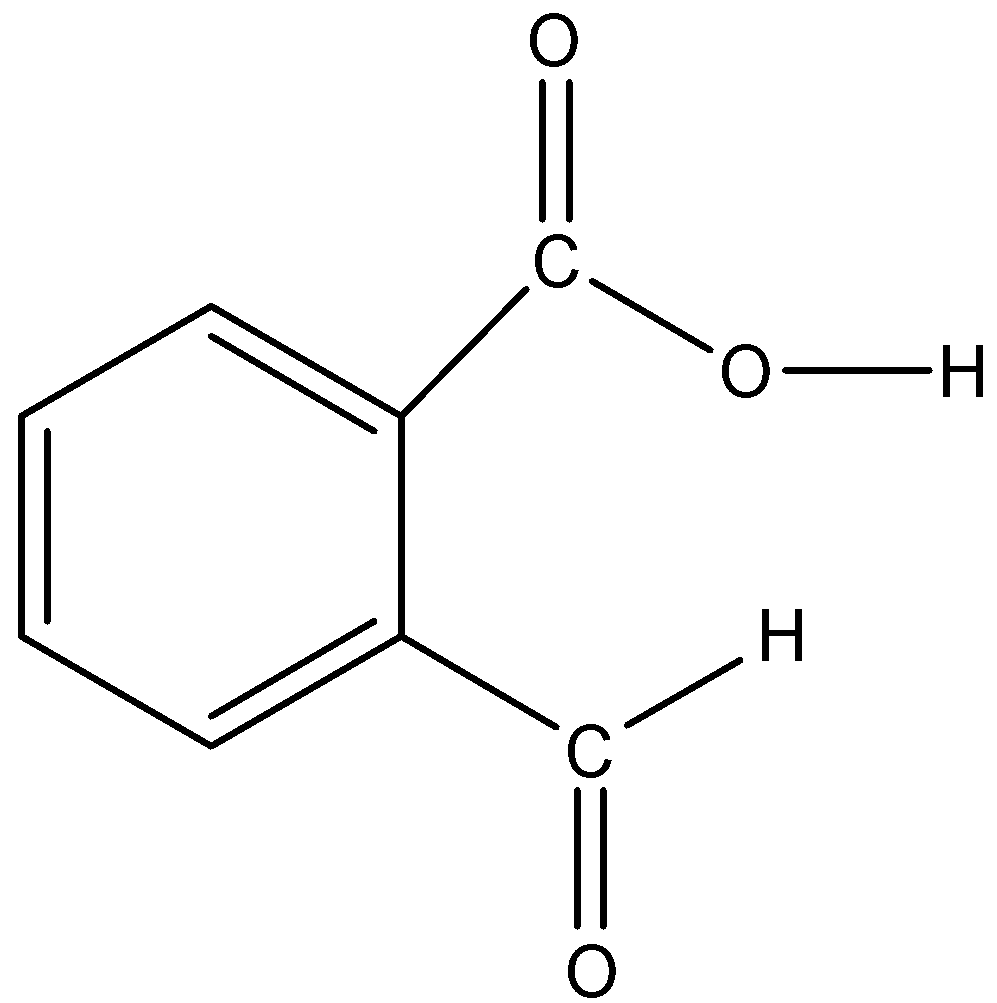
Therefore, the number of sigma bonds is 17 and the number of pi bonds is 5.
So, the correct answer is “Option (d)”.
Note:
The sigma bond is a stronger bond than the pi bond because overlapping in the sigma bond is along the internuclear axis but in the pi bond there is sideways overlapping of orbitals.
