Question
Question: The number of sigma bond in \[{{\text{P}}_4}{{\text{O}}_{10}}\] is:...
The number of sigma bond in P4O10 is:
Solution
We need to first draw the structure of P4O10. In the structure the valency of the element must get satisfied. Oxygen forms a maximum of 2 bonds and phosphorus can form a maximum of 5 bonds.
Complete step by step answer:
The structure of P4O10 is as follow:
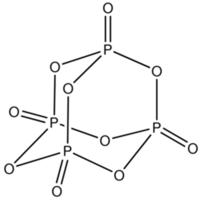
- A sigma bond is a single bond that is formed by the lateral overlapping between the atomic orbitals. Lateral overlapping is the overlapping that occurs along the internuclear axis. In the above molecule we have to find the number of sigma bonds. If there is only one bond present then it is always a sigma bond and if a double bond is present then one of them is sigma and the other one is a pi bond.
- In case a triple bond is present then one of them will be sigma and the other two will be a pi bond, however there is a triple bond in our formula. Now let us start counting the number of sigma bonds.
- As we can see that each phosphorus is linked with 3 other oxygen via a single bond only. So this makes the total number of phosphorous oxygen sigma bonds as 4×3=12.
- Now each phosphorus is having a double bond with oxygen. Out of this double bond one is single and so we have to count this as well. There will be 4 phosphorous oxygen single bonds or sigma bonds.
Hence, the total number of sigma bonds in P4O10 is 16.
Note:
The phosphorus pentoxide is basically P2O5. It exists in its dimer form formed by joining 2 molecules as P4O10.
