Question
Question: The number of sigma and pi bonds in peroxydisulfuric acid is/are: [If answer is \(13\) and \(6\), re...
The number of sigma and pi bonds in peroxydisulfuric acid is/are: [If answer is 13 and 6, represent as 136]
Solution
Sigma bonds are formed by the head on overlapping whereas pi – bonds are formed with sideways overlapping. Single bond is a sigma bond, in double bond there is 1σ or 1π - bond and in triple bond there is 1σ and 2π- bonds.
Complete step by step answer:
Peroxydisulfuric acid is the organic compound. It is also known as Marshall’s acid. It can also be said as the oxoacid of Sulphur.
Its chemical formula is H2S2O8
Molar mass: 194.14g/mol
Structure:
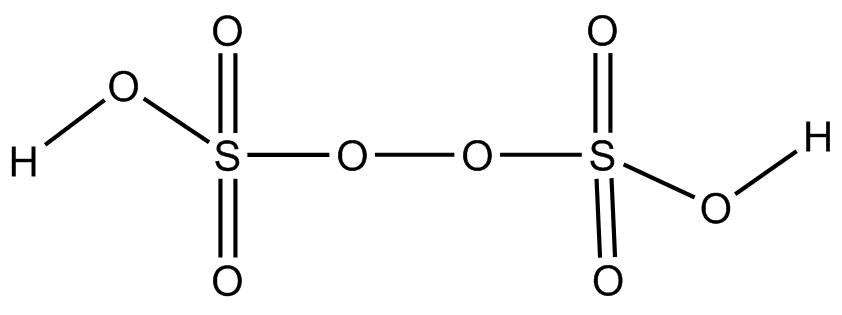
It is a colourless solid having no odor and is also soluble in water. The number of sigma or pi – bonds present in peroxodisulfuric acid are:
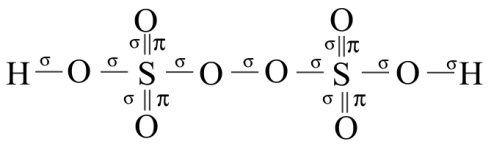
So, there are 11 sigma bonds and 4 pi – bonds in peroxodisulfuric acid.
As per the condition given in question the answer is 114.
Additional information:
(A) Peroxodisulphuric acid and its salts are used as a source of hydrogen peroxide and due to this the large scale production of sulphuric acid is possible.
(B) It is also used as a hypo – eliminator in photography
(C) It can also be used as a strong oxidant.
Note:
The sigma and pi bonds count should be made wisely by keeping in mind that both the double and triple bonds have only 1σ bond and the rest of bonds in double and triple bonds are pi – bonds.
