Question
Question: The number of \(\sigma \) and \(\pi \) -bonds in \(F{{e}_{2}}{{(CO)}_{9}}\) , respectively are: A....
The number of σ and π -bonds in Fe2(CO)9 , respectively are:
A. 22 σ and 15 π
B. 23 σ and 15 π
C. 22 σ and 16 π
D. 15 σ and 8 π
Solution
If p-orbitals are going to overlap axially then it leads to the formation of sigma (σ) bond and if p-orbitals are going to overlap sideways then it leads to the formation of the pi (π ) bond. Sigma bond is stronger than pi bond.
Complete answer:
- In the question it is given to find the number of sigma bonds and number of pi bonds present in the given compound.
- The given compound is Fe2(CO)9 .
- To know about how many sigma bonds and how many pi bonds present in the given compound we should know the structure of the given compound.
- The structure of the given compound is as follows.
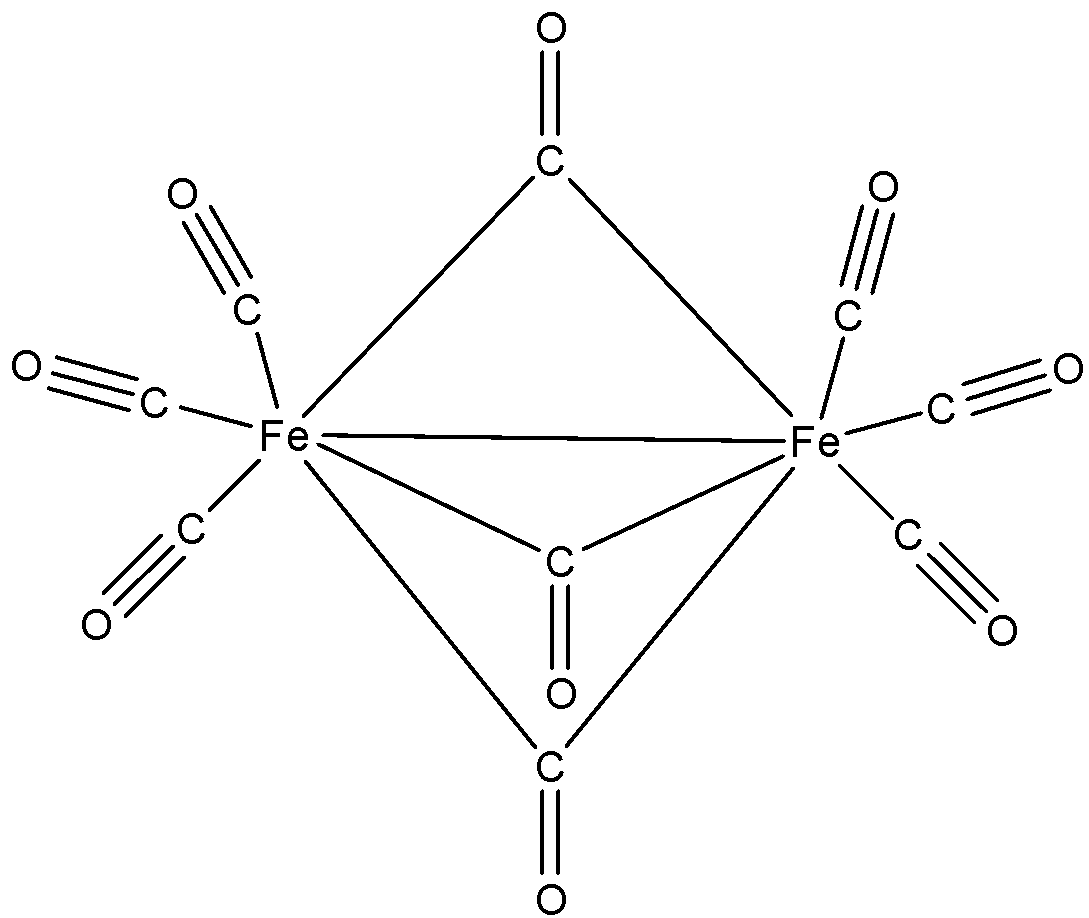
- All single bonds are sigma bonds only.
- In CO, there are three bonds. Out of those three one bond is sigma bond and the other two bonds are pi bonds.
- All Fe-CO bonds are sigma bonds in nature.
- If CO contains a double bond in the above structure then it contains one sigma bond and one pi bond in it.
- Therefore the number of sigma bonds present in the given compounds is 22 and the total number of pi bonds is 15.
So, the correct option is A.
Note:
Triple bonds are stronger than the double bond. Because in triple bonds there are two pi bonds and one pi bond while in a double bond there is one sigma bond and one pi bond. Therefore triple bonds are stronger than pi bonds.
