Question
Question: The number of sigma and pi bonds in benzene are: a. 6 sigma and 3 pi bonds b. 12 sigma and 3 pi...
The number of sigma and pi bonds in benzene are:
a. 6 sigma and 3 pi bonds
b. 12 sigma and 3 pi bonds
c. 9 sigma and 3 pi bonds
d. 6 sigma and 6 pi bonds
Solution
Benzene is a hydrocarbon composed of six carbons connected via covalent bond, and exists in the form of a ring. It has three alternating double bonds which makes the compound very stable.
Complete step by step answer:
Benzene is an aromatic compound of carbon and hydrogen. The molecular formula for benzene is C6H6.
Let us draw the structure of Benzene.
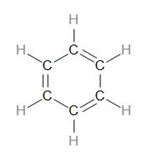
To calculate the number of sigma bonds in this, we can draw the skeletal structure by only drawing the sigma bonds –
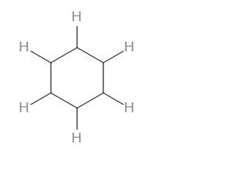
As we can see, there are 6 C-H bonds, 6 C-C bonds.
Therefore, we can say that there are a total of 12 sigma bonds in benzene.
Now, looking at the structure of benzene, we can see that there are 3 C=C bonds.
Therefore, there are 12 sigma bonds and 3 pi bonds. Benzene is therefore made up of 15 covalent bonds.
Therefore, the answer is – option (b) – The number of sigma and pi bonds in benzene are 12 and 3, respectively.
Additional Information:One single bond contains one sigma bond; a double bond contains one sigma and one pi bond.Similarly, a triple bond contains one sigma and two pi bonds.
Note: Benzene exists as a colorless and highly flammable liquid with a sweet smell. This sweet smell is produced as a result of aromaticity of the compound. Aromaticity of benzene arises due to the continuous cyclic pi bonds between the carbon atoms. It is a very stable compound. Benzene is naturally present in crude oil and is also an elementary petrochemical.
