Question
Question: The number of \[s{p^3} - s\] sigma bonds in benzene is: A.3 B.6 C.12 D.none...
The number of sp3−s sigma bonds in benzene is:
A.3
B.6
C.12
D.none
Solution
We must know that the sp3 configuration of C is having one double bond and two single bonds and all the carbons in benzene are sp3 hybridized.
Complete step by step answer:
Let’s start by understanding the structure of benzene first; in benzene each carbon atom is having one double bond and 2 single bond. The structure is hexagonal in shape and the molecular formula is C6H6. The structure of benzene is given below.
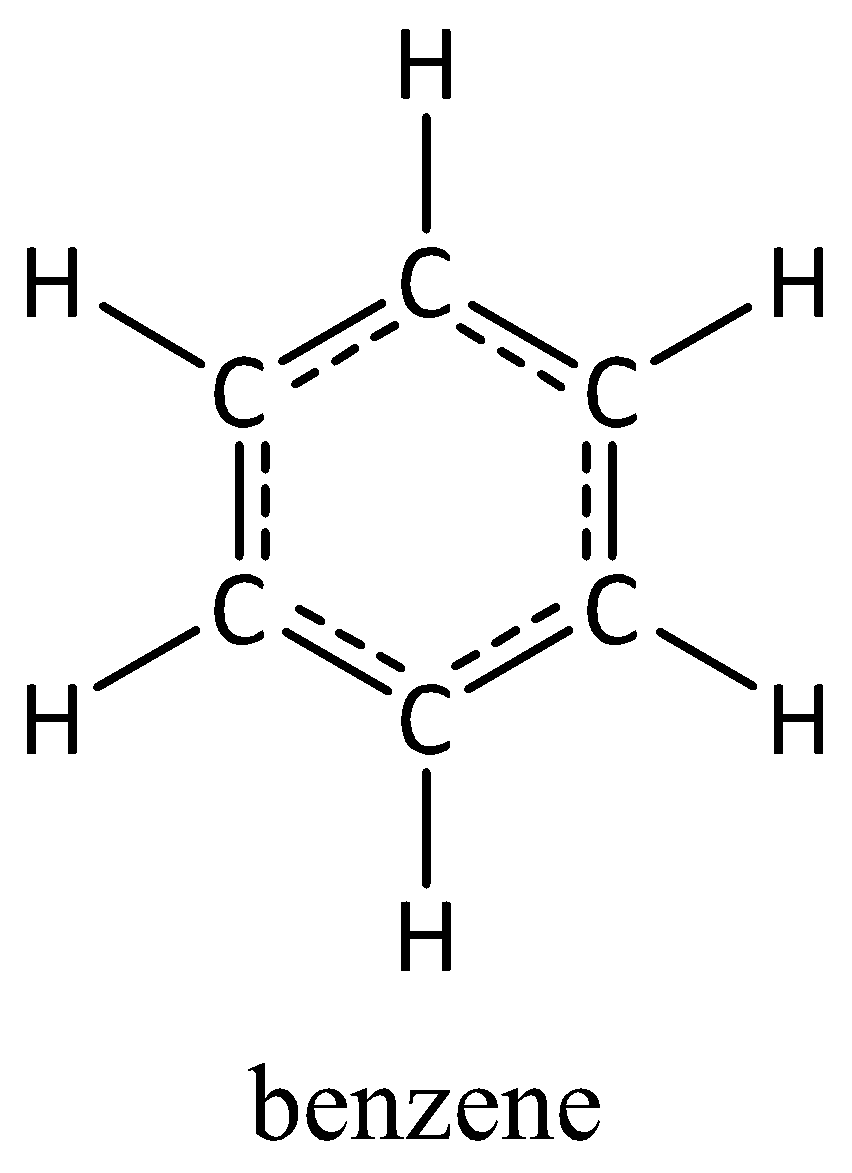
The sp3 configuration of C is having one double bond and two single bonds, so we can say each carbon atom in a benzene ring is sp3 hybridized. The hydrogen atom present in benzene is always in 1s hybridization. So, the bond formed between the C and hydrogen in benzene can be counted as sp3−s sigma bond.
Counting the number of C and H bonds in benzene, the number of bonds are 6. So, 6 sp3−s sigma bonds are present in benzene.
∴ The answer to this question is B i.e 6.
Note:
We must know that the hybridization gives an idea about how the atomic orbitals of atoms will fuse together and give rise to the bond. It gives an idea about how strong the bonds are between the two atoms. It also tells about the shielding effect that an orbital provides to the electron. The highest shielding effect is of the s orbital followed by p, d and at last the f orbital which is having the poorest shielding effect.
