Question
Question: The number of \({\rm{S}} = {\rm{O}}\) and \({\rm{S}} - {\rm{OH}}\) bonds present in peroxodisulfur...
The number of S=O and S−OH bonds present in
peroxodisulfuric acid and pyrosulfuric acid respectively are:
A) (4and2)and(2and4)
B) (2and4)and(2and4)
C) (4and2)and(4and2)
D) (2and2)and(2and2)
Solution
We know that the acid which contains oxygen in the compound, then this acid, is termed as oxyacid or oxoacid. The compound generally contains hydrogen, oxygen, and some other elements in the formula are necessary. The hydrogen bond dissociates and forms a hydrogen ion as a cation, and the oxygen bond dissociates to form an anion of the acid.
Complete step-by-step answer: As we know that, the peroxodisulfuric acid and pyrosulfuric acid are the examples of oxyacid and both contain sulfur oxygen and sulfur hydrogen bond. In the oxyacids, the oxygen is the main element.
The structure of peroxodisulfuric acid is shown below.
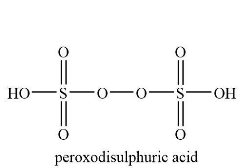
According to the structure of the peroxodisulfuric acid, it contains four S=O bond and two S−OH bond.
Similarly, the structure of pyrosulfuric acid is shown below.
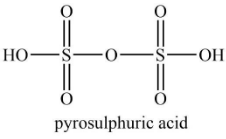
According to the structure of pyrosulfuric acid, it contains four S=O bond and two S−OH bond.
Therefore, the correct option for this question is C that is (4and2)and(4and2).
Note: The oxyacid is used to manufacture several dyes, explosives substances, and drugs. It is also termed as ternary compounds or acids. It is also used for the manufacture of several fertilizers. The oxyacid is used as laboratory reagent and used in the manufacture of other compounds.
