Question
Question: The number of possible alkynes with molecular formulae, \({{C}_{5}}{{H}_{8}}\) A) 1 B) 3 C) 5 ...
The number of possible alkynes with molecular formulae, C5H8
A) 1
B) 3
C) 5
D) 8
Solution
Different types of isomerism will give us different structures-position isomers and chain isomerism can yield us different structures. The number of bonds a C can form is four only. The general formula of alkynes is - CnH2n−2, n is the number of C atoms present in the compound.
Complete answer:
So here we are asked to give the number of alkynes that can be formed from the equationC5H8by satisfying the number of C and H given in formula.
So let’s start writing the possible structures.
There are five C atoms in the given formulae, so let’s write the possible C skeleton that could be drawn for five C atoms. The structures are as follows-
The straight chain-n-pentane
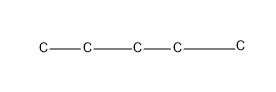
Then giving one C as the substituent, which is a chain isomer, isopentane.
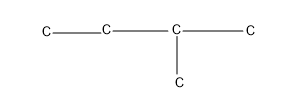
The next one giving two substituent, isomer which comes under neo compounds-neopentane
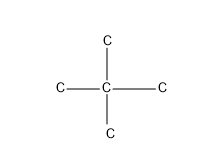
So these are the possible C skeleton for five Carbon atoms.
Now the question is about having alkynes, so let’s give a triple bond in the first carbon and yield an alkyne, pent-1-yne.
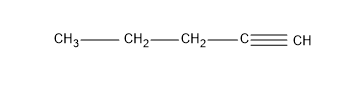
Then we can change the position of the triple bond and give the triple bond to the second carbon and we will get an alkyne, pent-2-yne
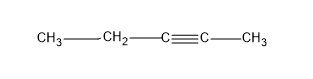
These two structures are examples for positional or position isomers. As the difference in the both structures is only the difference in the C position they are attached to.
And we got two structures, from the straight C chain, by changing the position of the triple bond.
If we again move the triple bond to next C then it will be similar, as in that case we will number from the right side of the molecule.
So now let’s move to the next C skeleton form having one substituent.
For that we will give one −CH3 (methyl group) as the substituent and let’s write the structure by satisfying the valency of C with H atom.
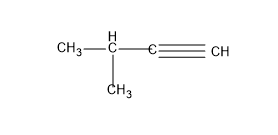
And this structure is called as -3-methyl but-1-yne.
No other structures are possible for this C skeleton. So let’s move to the next C skeleton with 2 groups as the substituent, but in this case it is not possible to give the triple bond since C can have only 4 bonds and in this type of C skeleton they will only possess a single bond.
So the structures we obtained are – pent-1-yne, pent-2-yne and 3-methyl but-1-yne i.e. in total of three structures.
So the correct option for the question is option (B).
Note: While writing the structure we should always satisfy the valency of the C atom and the number of C and H atoms in alkynes should satisfy the general formulae- CnH2n−2. If the question was for alkenes, the process should be continued through this method and they will have the general formulae -{{C}_{n}}{{H}_{2n}}$$$${{C}_{n}}{{H}_{2n}} and for alkanes it isCnH2n+2. There will be more structures possible for alkenes and alkanes than the alkynes.
