Question
Question: The number of peroxide bonds in perxenate ion \[{{[Xe{{O}_{6}}]}^{-4}}\] is: (A) 0 (B) 2 (C) 3...
The number of peroxide bonds in perxenate ion [XeO6]−4 is:
(A) 0
(B) 2
(C) 3
(D) 1
Solution
Peroxide bond means, a compound in which two oxygen atoms are attached together by a single bond. A number of organic and inorganic peroxides are available. The compounds that have a peroxide bond are called as peroxides.
Complete step by step answer:
Now we have to find the number of peroxide bond present in the given molecule perxenate ion is [XeO6]−4.
The structure of the [XeO6]−4is as follows.
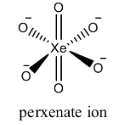
Perxenate ion has six oxygen atoms and is connected to central metal xenon atom. The structure of perxenate ions is regular octahedral.
Two oxygen atoms are attached to xenon atoms through double bonds and the remaining four hydrogen atoms are attached to xenon through single bonds.
But we cannot see any peroxide bonds in the above structure.
Therefore the number of peroxide bonds in the given molecule is zero.
So, the correct option is A.
Additional information:
Peroxides can be used as bleaching agents, and as initiators in the polymerization reactions to prepare polymers.
Peroxides are used in the preparation of hydrogen peroxide and other oxygen compounds.
[XeO6]−4can be used to separate minute amounts of americium from curium.
Maximum metal perxenates are stable excluding silver perxenate.
Note: Perxenic acid also called as perxenate ion ([XeO6]−4). It is a strong oxidizing agent and it is capable of oxidizing silver (I) to silver (III), and copper (II) to copper (III). The perxenate ion is unstable in acidic solutions and very rapidly reduced to[HXeO6]−.
