Question
Question: The number of oxides which is/are more basic as compared to \[N{a_2}O\] is/are: \[L{i_2}O,{K_2}O,...
The number of oxides which is/are more basic as compared to Na2O is/are:
Li2O,K2O,Cs2O,Rb2O,MgO
Solution
The group one element consists of lithium, sodium, potassium, rubidium, cesium, and francium. These elements are known as alkali metals as they react strongly with water and form hydroxide ions and hydrogen gas. Lithium reacts with oxygen to give lithium oxide, potassium gives potassium oxide, rubidium gives rubidium oxide and cesium gives cesium oxide. Magnesium belongs to the group of alkaline earth metals and reacts with oxygen to give magnesium oxide.
Complete step by step answer:
As we go down the group the atomic size of elements increases hence their tendency to donate electrons also increases. This`` means that metallic character also increases hence the basicity of oxides as we go down the group also increases.
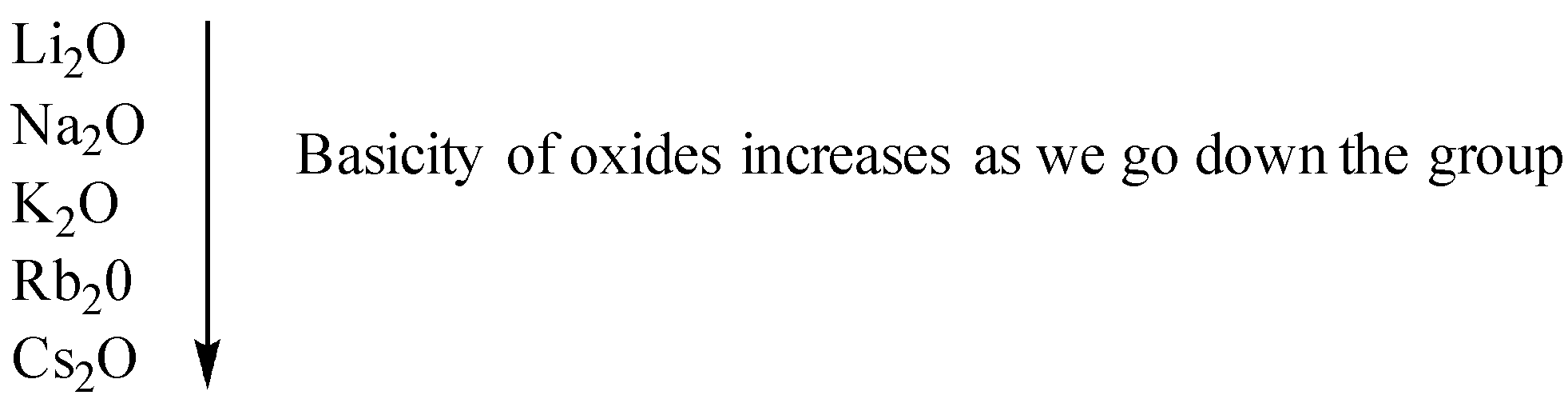
As we move along the period the non-metallic character increases because the atomic size decreases as we move along the period hence the basic character of oxides decreases as we move along the period.
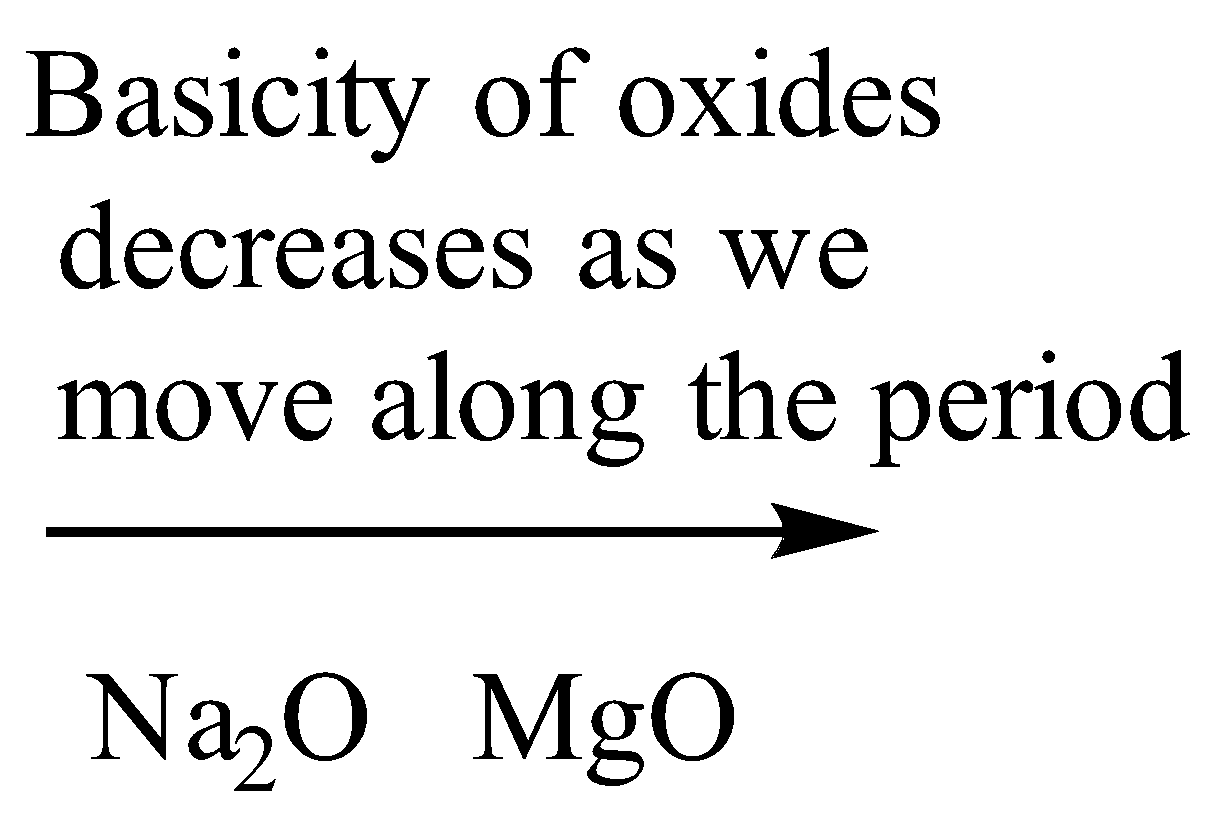
Therefore, from the above representation of the periodic table, we can see that oxide of potassium, Rubidium, and cesium is more basic as compared to sodium as they lie below it in the periodic table. Magnesium lies on the right to sodium in the same period hence it is less basic.
Therefore, the correct answer is 3 (K2O,Rb2OandCs2O)
So, the correct answer is Option A.
Note: Metals form basic oxides whereas non-metals lead to the formation of acidic oxides.
Metallic character increases as we move down the group whereas it decreases as we move from left to right along a period
Nonmetallic character decreases down the group and increases along a period.
The reactivity of metals also increases down the group due to a decrease in ionization enthalpy.
