Question
Question: The number of moles of KI required to produce 0.1 moles of \[{{K}_{2}}[Hg{{I}_{4}}]\]is? A. 1.6 ...
The number of moles of KI required to produce 0.1 moles of K2[HgI4]is?
A. 1.6
B. 0.8
C. 3.2
D. 0.4
Solution
-To produce K2[HgI4]from KI we need a chemical. The required chemical is HgCl2 (mercuric chloride).
-The name of the KI is potassium Iodide.
-The name of theK2[HgI4]is potassium mercuric iodide.
-The colour of the potassium mercuric iodide is yellow.
Complete step by step solution:
-The formation of K2[HgI4] from KI is a two-step process.
Step-1: Potassium iodide reacts with HgCl2 (Mercuric chloride) and forms HgI2 (Mercuric Iodide).
2KI+HgCl2→HgI2+2KCl
Step-2: The formed HgI2 (Mercuric Iodide) reacts with excess amount of KI and forms potassium mercuric iodide (K2[HgI4]).
HgI2+2KI→potassium mercuric iodideK2[HgI4]
-The overall reaction can be written as follows.
4KI+HgCl2→K2[HgI4]+2KCl
-From the above equation we can say that four moles of potassium iodide reacts with Mercuric iodide and forms one mole ofK2[HgI4](potassium mercuric iodide).
1 moles of K2[HgI4] requires 4 moles of KI
0.1 moles of K2[HgI4] requires = 0.1 (4) = 0.4 moles of KI.
-Therefore to produce 0.1 moles of K2[HgI4], there is a requirement of 0.4 moles of KI.
So, the correct option is D.
Additional information:
- Potassium mercuric iodide is a yellow colour solid and it is odourless.
-Potassium mercuric iodide is a salt and it is used as Nessler's reagent.
- Potassium mercuric iodide is soluble in water.
-The colour of the alkaline Potassium mercuric iodide is pale orange.
Note: Potassium mercuric iodide is used widely to determine the number of ammonium compounds. The structure of Potassium mercuric iodide is as follows.
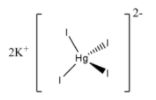
0.09mol/L Potassium mercuric iodide in 0.25mol/L potassium hydroxide is called Nessler’s reagent.
