Question
Question: The number of moles of HCHO required to convert I mole of X completely into Y when X is treated with...
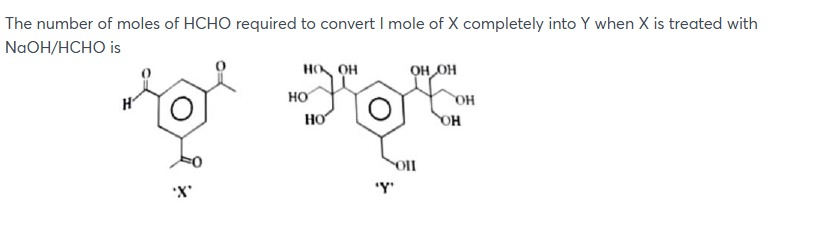
The number of moles of HCHO required to convert I mole of X completely into Y when X is treated with NaOH/HCHO is
1
2
3
5
5
Solution
The reaction involves two main transformations:
-
Reduction of the aldehyde group: The aldehyde group (-CHO) in X is converted to a hydroxymethyl group (-CH2OH) in Y. This is achieved via a crossed Cannizzaro reaction where formaldehyde is oxidized to formate, and the aldehyde of X is reduced. This step consumes 1 mole of HCHO per mole of X.
-
Reaction of the acetyl groups: Each acetyl group (-COCH3) in X is converted into the substituent -C(CH2OH)(OH)-CH(OH)-CH2OH in Y. This transformation involves the reaction of the carbonyl carbon and the alpha-methyl group with formaldehyde. The structure of the substituent in Y suggests that each acetyl group reacts with 2 molecules of formaldehyde. This accounts for the addition of two carbons and the formation of multiple hydroxyl groups. Therefore, for the two acetyl groups in X, a total of 2 * 2 = 4 moles of HCHO are consumed.
Total moles of HCHO required = (moles for aldehyde reduction) + (moles for acetyl group transformation) Total moles of HCHO = 1 + 4 = 5 moles.
