Question
Question: The number of moles of \(BaC{O_3}\), which contains \(6\) mol of oxygen (atom), is:...
The number of moles of BaCO3, which contains 6 mol of oxygen (atom), is:
Solution
BaCO3 is the chemical formula of Barium carbonate which is an inorganic compound. It is poorly soluble in water and like other alkali metals, it is a white salt. BaCO3 is mainly used to remove sulfate impurities from feedstock of the chlor-alkali process. It is also used in ceramic industries and also acts as a flux, a matting and crystallizing agent and combines with certain coloring oxides to produce colors which are not easily attainable.
Complete step by step answer:
1 mol of BaCO3 contains 3 moles of oxygen. So we can find out the number of moles of BaCO3 present in 6 moles of oxygen just by dividing the number of moles of oxygen total number of oxygen moles present in one mole of BaCO3.
1 Mol of BaCO3 contains 3 moles of oxygen
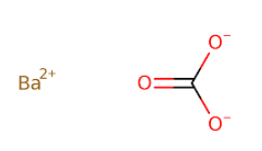
Fig: Chemical structure of BaCO3.
∴6 moles of oxygen present in 1 mol of BaCO3 =36=2.
Therefore, the number of moles of BaCO3 is 2.
Note:
The above formula can also be used in finding the relation between the number of moles and molar mass.
Number of moles=mass/relative formula mass.
This can also be used in finding the molar mass if the mass and number of moles are known.
