Question
Question: The number of lone pairs and bond pairs in hydrazine are respectively: (A) 2 and 4 (B) 2 and...
The number of lone pairs and bond pairs in hydrazine are respectively:
(A) 2 and 4
(B) 2 and 6
(C) 2 and 5
(D) 1 and 5
Solution
Hydrazine is a colorless liquid with an ammoniacal odor and the hydrazine is miscible with water in all proportions .The molecule N2H4is called hydrazine. From drawing the Lewis structure ofN2H4, we could identify the number of bond pairs and lone pairs of electrons in hydrazine.
Complete answer:
- As we mentioned hydrazine is an inorganic compound with the molecular formulaN2H4. It’s a flammable and colourless liquid with an ammonia like odour.
- N2H4 belongs to a series of compounds called hydro nitrogens and it’s a powerful reducing agent. It can readily absorb moisture and the hydrate N2H4.H2O is formed. N2H4 reacts with metallic salts and acids and the products formed are used in the manufacturing of fungicides and explosives.
- Hydrazine is also found in human liver and kidney issues and it’s also detected in certain body fluid such as blood and urine. N2H4 also reacts with organic compounds to give alkyl hydrazine which is used in jet and rocket propulsions .
- N2H4 is a strong reducing agent and a highly reactive base and it’s also known as diamine belonging to the class of inorganic compounds known a homogenous other nonmetal compounds.
- The Lewis structure of hydrazine molecule is illustrated below
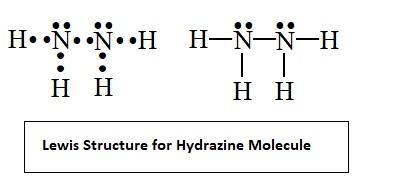
- From the Lewis structure it’s clear that there are five bond pairs which includes the one bond between the two nitrogen atoms and four bonds present between the hydrogen and nitrogen atoms. Also there are two lone pairs on nitrogen (one lone pair on each nitrogen atom).
Therefore the number of bond pairs and lone pairs of electrons in hydrazine molecules are 5 and 2 respectively.
Thus the answer is option (C) 2 and 5.
Note:
Keep in mind that the bond between nitrogen atoms in hydrazine is single, not a triple bond. It’s for the reason that we only have one p-orbital per nitrogen atom that is available to form a pi-bond, and in order to form two pi-bonds we need at least two p-orbitals per each nitrogen atom to be available as in the case of acetylene.
