Question
Question: The number of isomers of \({C_5}{H_{10}}\) is: A. \(10\) B. \(11\) C. \(12\) D. \(13\)...
The number of isomers of C5H10 is:
A. 10
B. 11
C. 12
D. 13
Solution
When more than one compound has the same molecular formula but different structural formula, then the compounds are known as isomers and the phenomenon is known as isomerism. It is broadly divided into two types i.e., structural isomerism and stereoisomerism.
Complete answer:
To find the isomers of given organic compound, we first need to find the degree of unsaturation which indicates the total number of pi bonds and ring possible within a structure and it is calculated as follows:
Degree of unsaturation (n) =C−2H−2X+2N+1
Where, C is the number of carbon atoms, X is the number of hydrogen atoms, X is the number of halogen atoms, N is the number of nitrogen atoms within the molecular formula of the given organic compound.
Therefore, the degree of unsaturation for C5H10 will be as follows:
n =5−210+1
⇒ n =1
The degree of unsaturation is one which indicates that only one double bond is present in the straight chain structure and one complete ring (i.e., without substitution) is possible.
Hence, the possible isomers for C5H10 are as follows:
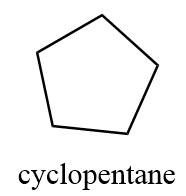
1. cyclopentane
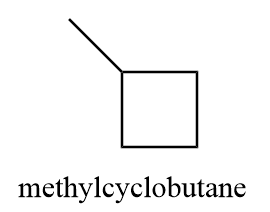
2. methylcyclobutane
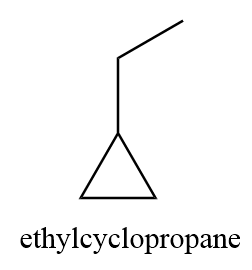
3. ethylcyclopropane
4. 1,1-dimethylcyclopropane
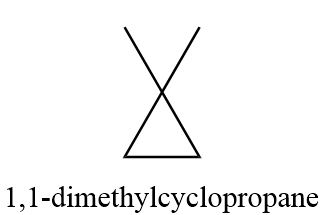
5. (1R,2S)-1,2-dimethylcyclopropane
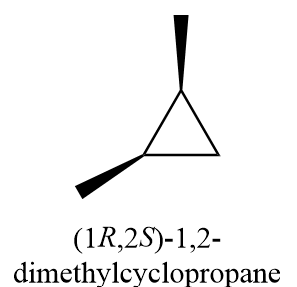
6. (1R,2R)-1,2-dimethylcyclopropane
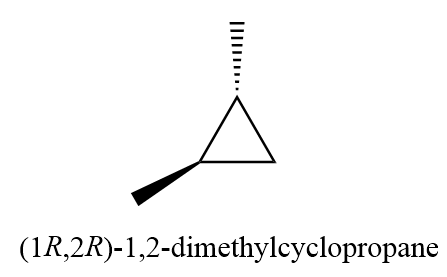
7. (1S,2S)-1,2-dimethylcyclopropane
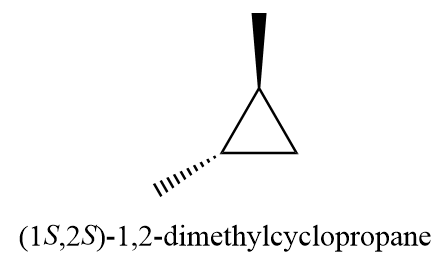
8. pent-1-ene
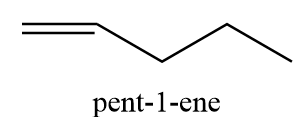
9. (Z)-pent-2-ene
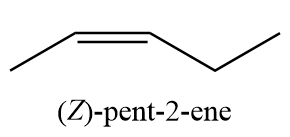
10. (E)-pent-2-ene
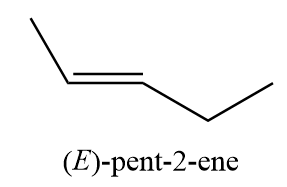
11. 2-methylbut-1-ene
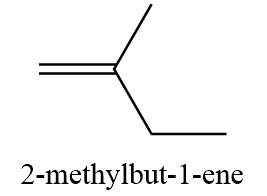
12. 3-methylbut-1-ene
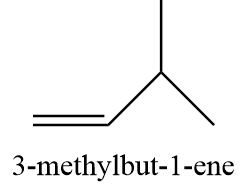
13. 2-methyl-but-2-ene
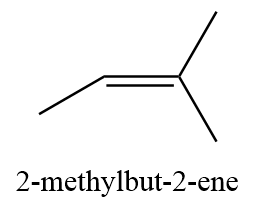
Therefore, the number of possible isomers (including stereoisomers) for C5H10=13.
So, option (D) is the correct answer.
Note:
It is important to note that there is no specific formula to calculate the number of isomers possible for an organic compound. You need to draw each possible structure to find the number of isomers. Moreover, we can find the number of geometrical isomers possible for an unsaturated organic compound which is equal to 2n, where n is the number of double bonds or degree of unsaturation of the compound.
