Question
Question: The number of isomers in \({C_4}{H_{10}}O\) are: a.)7 b.)8 c.)6 d.)5...
The number of isomers in C4H10O are:
a.)7
b.)8
c.)6
d.)5
Solution
Draw the different structures possible using this formula. The number is the sum of isomers both with alcohol as a functional group and ether.
Complete step by step answer:
This question is about the number of isomers in C4H10O. First, let’s see what structural isomers are:
The structural isomers are those in which atoms remain same while they differ in bonding with each other. The number of atoms of each type are the same. Now, for the compound given to us which is a four-carbon compound with one Oxygen atom. We know that for such a compound two functional groups are possible which are alcohol and ether.
Now, let’s start drawing the structure of alcohol first.
The first molecule, we will draw with all four carbons in a single chain. It will be n- Butanol.
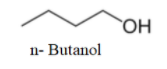
We can see that one more molecule with straight chain can be drawn as-
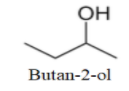
Now, as no more straight chain molecule can be formed; so, we will make branched molecules as-
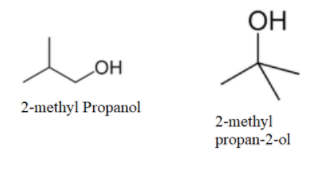
We can see that no more molecule with alcohol as a functional group can be made. So, now we will make ethers as-
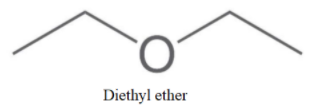
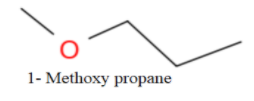
Now, no more straight chain ether is possible, So, we will form branching as-
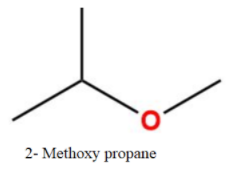
Thus, the total number of isomers are-
Alcohols=4
Ethers=3
Total isomers=7
So, option a.) is the correct answer.
Note:
If we try to make more number of straight chain alcohol, it will form the same molecule only. Example is-
Both these will form one molecule only.

