Question
Question: The number of hydrogen atoms present in 25.6 g of sucrose \[({C_{12}}{H_{22}}{0_{11}})\] which has a...
The number of hydrogen atoms present in 25.6 g of sucrose (C12H22011) which has a molar mass of 342.3g is;
A. 22×1023
B. 9.91×1023
C. 11×1023
D. 44×1023
Solution
The common example of a disaccharide is sucrose. Sucrose contains glucose and fructose in one molecule. saccharides are a group of organic compounds. The general formula of saccharide is CnH2nOn . The ratio of hydrogen and oxygen is always 2:1 . Saccharides are also called carbohydrates. This is biomolecules.
Complete step by step answer:
Based on the number of saccharide molecules attached saccharides are classified into four different types These types are,
Monosaccharides, disaccharides, trisaccharide, and polysaccharides, etc. sucrose is a disaccharide.
The structure of sucrose is shown below.
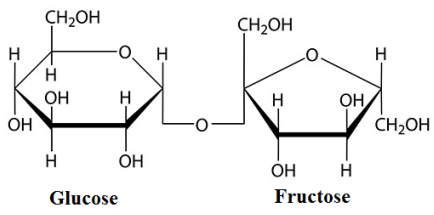
In this structure, the bonding between glucose and fructose is called glycosidic linkage. And this glycosidic linkage is at C1 carbon of glucose and C5 carbon of fructose.
Molecular mass means the weight of the 1-mole amount of a compound. And according to the mole concept, we know 1 mole of any substance is equal to its molecular weight. And 1 mole is also equal to Avogadro’s number of atoms i.e. 6.023×1023 .
Therefore, 342.3g sucrose contains 6.023×1023 a number of sucrose molecules.
Each sucrose has 22 numbers of hydrogen atoms. Therefore, 342.3g sucrose contains 22×6.023×1023 a number of hydrogen atoms.
Now, according to the question: The number of hydrogen atoms present in 25.6 g of sucrose (C12H22011) is,
So, the correct option is B.
Note: Remember that Sucrose contains glucose and fructose in one molecule. Based on the number of saccharide molecules attached saccharides are classified into four different types. There is another classification of saccharides based on the reducible character of the saccharide. Sucrose is a reducible saccharide.
