Question
Question: The number of groups which can lead to the formation of an oxime when Q is treated with hydroxylamin...
The number of groups which can lead to the formation of an oxime when Q is treated with hydroxylamine (excess) at pH=5, is:
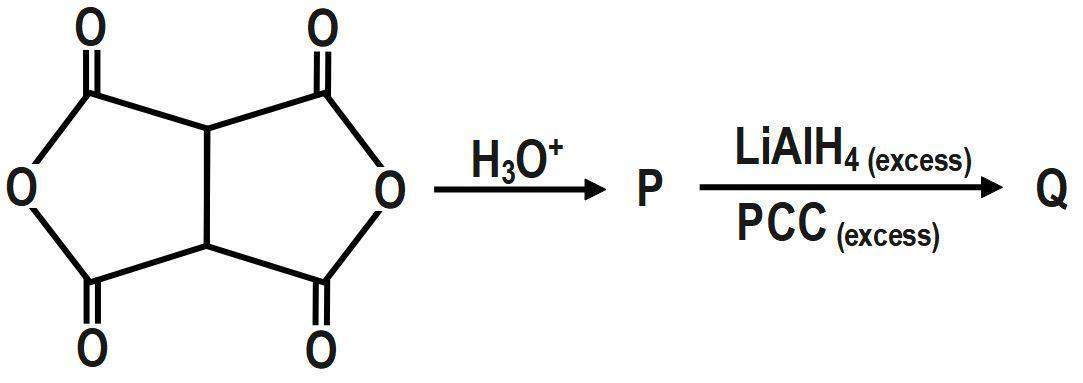
Solution
Hint : We know that the functional group that possesses a double bond of Carbon and Nitrogen is termed as imine. The nitrogen atom further can be bonded to an organic group to hydrogen (H). It is considered a Schiff base if the group to which the N atom is bonded is not a hydrogen atom. Oxime is a compound that belongs to this class.
Complete Step By Step Answer:
As we know that due to the minimal rotation of C=N bond, syn and anti-configuration, the oxymes demonstrate Isomerism. To find the isomerism one should know the reaction of above compounds in hydroxylamine. Isomerism occurs where atoms or groups are arranged uniquely in space due to the limited rotation of bonds or bonds in a molecule. Due to the minimal rotation of the C=N bond, the oxymes exhibit Isomerism.
To differentiate them, the descriptors, syn, and anti, are used. The syn form is where both the hydrogen and the hydroxyl (−OH) group are on the same side of the C=N in the case of aldoximes whereas in the anti-form, they are on the opposite side. Syn and anti-descriptors, however, suggest the spatial association between the first group referred to in the name and the hydroxyl group of ketoximes. The accompanying butanone ketoxime, for example, may be referred to as either syn methyl ethyl ketoxime or anti-ethyl methyl ketoxime. The following number of groups which can lead to the formation of an oxime when Q is treated with hydroxylamine (excess) at pH=5, is:
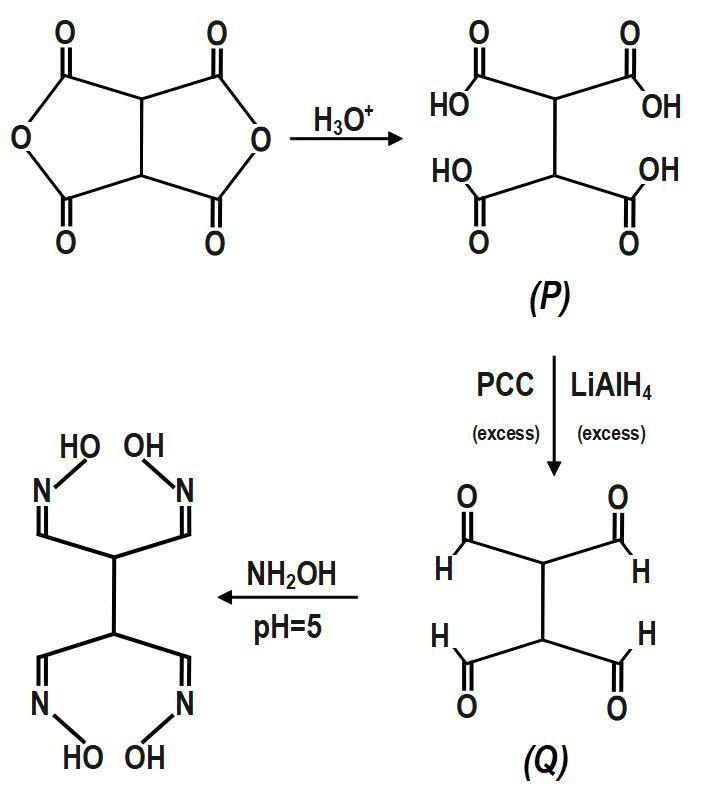
Note :
Remember that there are many industrial uses of oxime, such as production of caprolactam (monomer of nylon−6 ), catalytic uses in industry etc. Use of compounds of oxime as antidotes is also one of its uses. Acetone oxime is also used as a corrosion inhibitor.
