Question
Question: The number of equivalent \[Cl - O\] bonds in \(C{l_2}{O_7}\) is: A. \(6\) B. \(4\) C. \(8\) ...
The number of equivalent Cl−O bonds in Cl2O7 is:
A. 6
B. 4
C. 8
D. 2
Solution
A chemical substance composed from the atoms of different elements which are bonded together by chemical bonds is known as chemical compound. Dichlorine heptoxide is a chemical compound, chlorine and oxygen elements have contributed to this compound.
Complete step by step answer:
A chemical substance composed of many molecules made up of atoms of more than one element which are held together by chemical bonds is known as a chemical compound. If it is made up of a single type of element then it is not called as a compound rather it is a molecule.
Dichlorine heptoxide is a chemical compound composed of oxygen and chlorine element atoms. The chemical formula of dichlorine heptoxide is Cl2O7. Dichlorine heptoxide is basically the anhydride of perchloric acid. When maximum possible water molecules are removed from the perchloric acid, dichlorine heptoxide is produced. The dehydrating agent used in this distillation process is phosphorus pentoxide.
Structure of dichlorine heptoxide (Cl2O7) is:
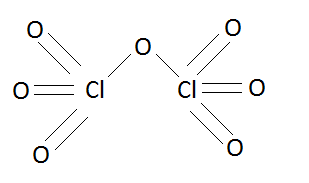
In the above structure we can see that one oxygen atom is shared by both of the chlorine atoms and rest oxygen atoms are distributed equally. This sharing of oxygen atoms or the linkage between three atoms is known as peroxy linkage.
So in this structure dichlorine heptoxide has two chlorine oxygen bonds (Cl−O) which are situated in the middle of this chemical substance.
Hence the number of equivalent Cl−O bonds in Cl2O7 are two.
So, the correct answer is Option D .
Note:
Basically dichlorine heptaoxide is an unstable compound or we can say it is an endergonic material which means intrinsic unstable. Easily it decomposes into its constituent elements with release of energy.
