Question
Question: The number of enantiomers of camphor is 
A. four
B. three
C. two
D. one
Solution
Enantiomers are optical active compounds. The presence of a chiral centre is necessary for a compound to be optically active. The maximum valency shown by carbon is four. A carbon having four different substituents is known as a chiral centre. We will determine the chiral centre then we will use the formula to calculate the number of enantiomers.
Complete solution:
The compounds that are mirror images of each other and are non-superimposable are known as enantiomers. Both enantiomers of an enantiomeric pair rotates the light in opposite directions. The enantiomer that rotates the light in a clockwise direction are known as dextrorotatory and represented by (+) sign and the enantiomer that rotate the light in an anticlockwise direction are known as laevorotatory and represented by (−) sign.
A carbon attached with four different types of groups is known as a chiral centre.
The given structure of the camphor is and chiral centre are shown as follows:
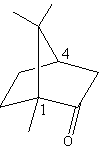
Carbon-one and carbon-fourth both are chiral centres as both carbon atoms have four different substituents.
So, the number of chiral centres in camphor is 2.
The formula to determine the number of enantiomers is as follows:
No. of enantiomers = 2n−1
Where,
n is the number of chiral centres.
On substituting 2 for n we get,
= 22−1
= 21
= 2
So, the number of enantiomers of camphor is 2.
The enantiomers of camphor are shown as follows:
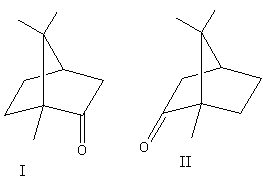
Both above structures are mirror images of each other and are non-superimposable.
Therefore, option (C) two, is correct.
Note: The compounds that are not mirror images of each other and have different configurations are known as diastereomers. The total number of stereoisomers is calculated by the formula 2n for an asymmetrical structure. If the molecule exists in two enantiomers and both are present in equal amounts and both rotate the light in opposite directions. This type of mixture when two enantiomers of a chiral molecule present in the same amount and both rotate the light in opposite directions is known as a racemic mixture.
