Question
Question: The number of electrophiles is: (I)- \(PC{{l}_{5}}\) (II)- \(I{{F}_{7}}\) (III)- \(B{{F}_{3}...
The number of electrophiles is:
(I)- PCl5
(II)- IF7
(III)- BF3
(IV)- CO2
(V)- C..Cl3
(VI)- 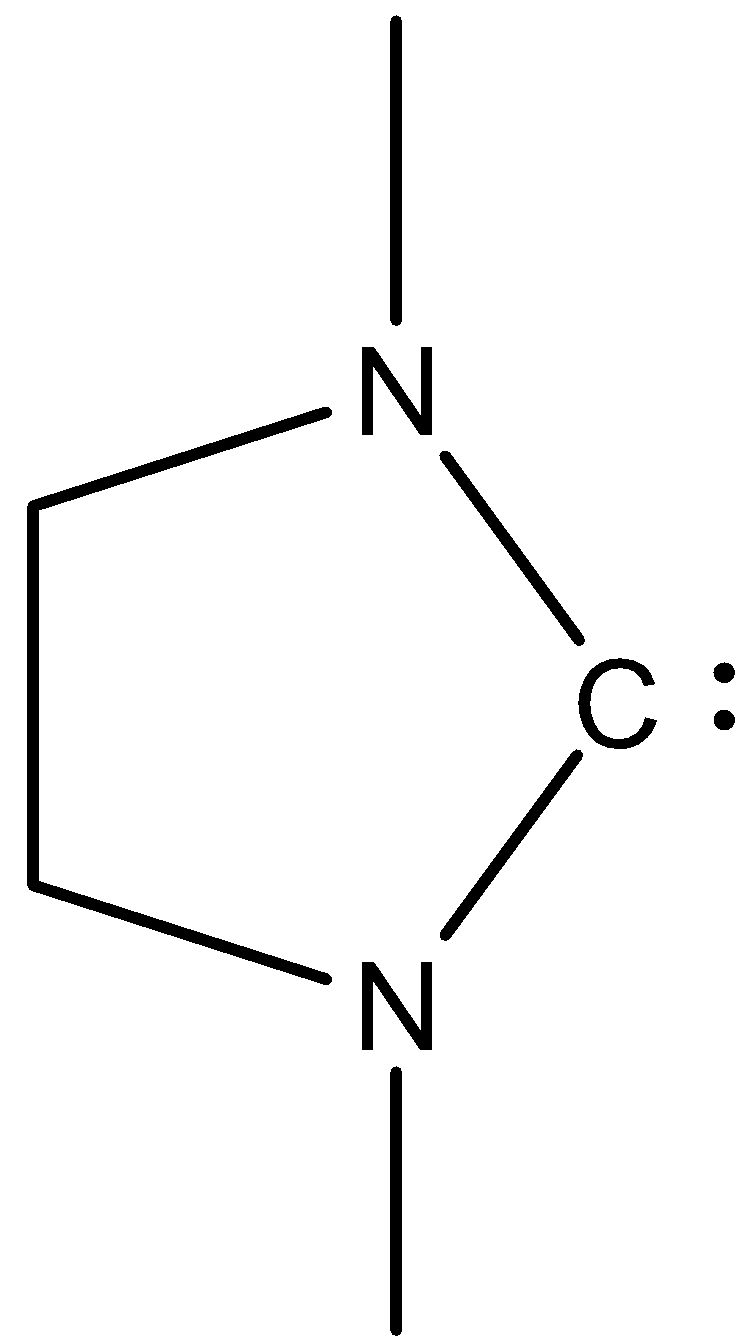
(VII)- C6H5−C.H2
(VIII)- C˙F3
Solution
The compounds that have less electron or those compounds are electron-deficient are known as the electrophiles. So, those who can accept a pair of electrons are electrophiles. Free radicals are also electrophiles.
Complete answer:
In organic reactions, when the reactant is attacked by any electrophiles or neutrophiles there is the formation of a product.
Now, electrophiles are those in which the numbers of electrons are less which affects the octet rule. The compounds that have less electrons or those compounds are electron-deficient are known as the electrophiles. So, those who can accept a pair of electrons are electrophiles. Free radicals are also electrophiles. Therefore, we can say that the compounds that are electrons loving species are electrophiles. Now let us see all the options:
(I)- PCl5: The phosphorus has 5 electrons in its valence shell and five chlorine atoms are attached with phosphorus, so this molecule is complete.
(II)- IF7: The iodine has 7 electrons in its valence shell and seven fluorine atoms are attached to the iodine, so this molecule is also complete.
(III)- BF3: The boron has 3 electrons in its valence shell and three fluorine atoms are attached to the boron, so there are a total of 6 electrons involved in this compound. Therefore, 2 electrons can be accommodated in the molecule. Hence, it is an electrophile.
(IV)- CO2: In carbon dioxide, there are two oxygen atoms which are electronegative atoms due to which the oxygen atom acquires a partial negative charge and the carbon atom acquires a partial positive charge, so it can act as an electrophile.
(V)- C..Cl3: In this compound the carbon atom has a total of eight electrons, so it is a complete molecule.
(VI)- 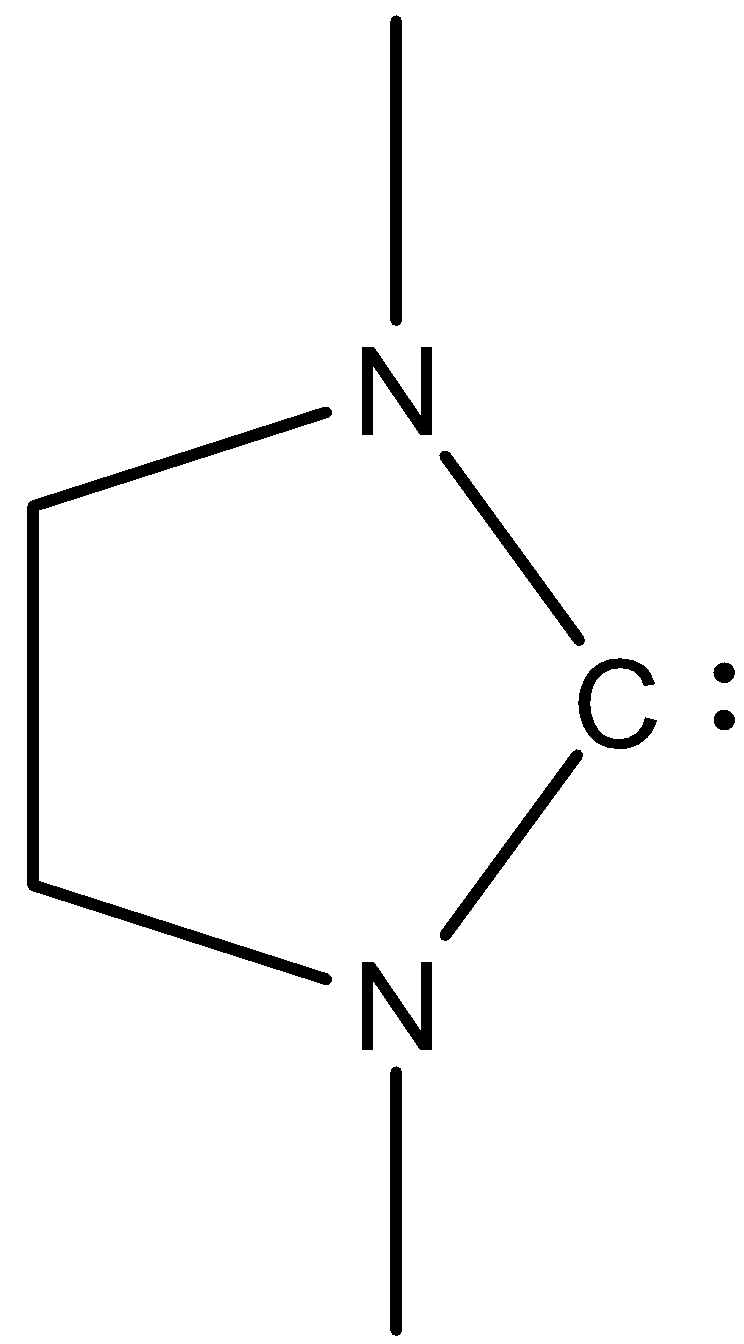
In this, the carbon atoms have six electrons, so it can accommodate two electrons, therefore it will be an electrophile.
(VII)- C6H5−C.H2: This compound is a free radical, so it will be an electrophile
(VIII)- C˙F3: This compound is a free radical, so it will be an electrophile.
Therefore, the number of free radicals are 5.
Note:
It can be noted that the electrophiles can accommodate electrons in its shell, so it will act as a Lewis acid. These electrophiles will attack the substrate molecule at the site of high electron density.
