Question
Question: The number of electron dots in Lewis structure indicates: A. Number of valence electrons in an ato...
The number of electron dots in Lewis structure indicates:
A. Number of valence electrons in an atom.
B. Number of protons in the nucleus of the atom.
C. Total number of electrons in an atom.
D. Total number of electrons and protons in an atom.
Solution
Lewis structure is also known as Lewis dot structure, electron dot structure or Lewis electron dot structure and Lewis structure diagram and this name is given to these structures by the name of the scientist Gilbert Newton Lewis who discovered this.
Step by step answer:
Basically Lewis dot structure is used to show the accurate structure of any molecule, because by the use of this structure we can describe the bonding between different atoms which are present within the molecule and these atoms will be denoted by their chemical symbol.
As we know that formation of bonds takes place by the sharing or donation of electrons from the outermost shell or valence shell.
So that in Lewis dot structure dots represent only the number of electrons that are present in the valence shell of an atom i.e. option (A) is correct.
Additional information: Lewis dot structure of SO3&NH3 is as follow, in which dot present on oxygen, sulphur, nitrogen and hydrogen represents the number of valence electron on that atom.
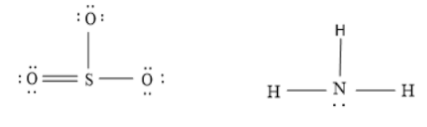
Note: For making Lewis dot structure always you should know about the atomic number and electronic configuration of atoms that are present within the molecule because when you have no knowledge about these two things then you never calculate no. of electrons that are present in the outermost or valence shell of atom.
