Question
Question: The number of double bonds present in the Lewis structure of \( {C_3}^{ - 4} \) is...
The number of double bonds present in the Lewis structure of C3−4 is
Solution
Hint : Draw the skeletal structure of the given compound first and then write the lone pairs around the atoms to satisfy their octet. In the given molecule 3 carbons are bonded together with two double bonds and there are 4 negative charges present. The bonds between them are covalent.
Complete Step By Step Answer:
Lewis structures are the simple representations of the valence shell electrons in a molecule. It is used to show the arrangement of electrons around individual atoms in a molecule. Structures are shown by drawing dots representing electrons or lines representing bonds between the two atoms.
Let us see how these structures are drawn:
First, count the total number of valence electrons in an atom. For anions, one electron will be subtracted for every negative charge. In the case of cations, one electron will be added for every positive charge.
In the second step, the skeletal structure of the molecule or ion will be drawn, and arrange the surrounding atoms around the central atom. Each atom will be connected to the central atom with a single bond first.
The third step, write the remaining electrons as lone pairs on the terminal atoms. The remaining electrons will be such that the octet of each atom is completed.
Place all remaining electrons on the central atom, and finally,if there are not enough electrons then form multiple bonds. and thus your Lewis structure is ready.
Now using the rules let us draw the Lewis dot structure of C3−4 .
It seems that there are three C atoms bonded to each other, and there are 4 negative charges on them so the Lewis structure of C3−4 will be as follows:
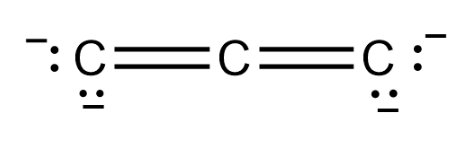
Therefore there are two double bonds in C3−4
Note :
It is important to write formal charges as Lewis dot structures are incomplete without the formal charges. Always remember to add lone pairs and check whether the octet rule of every atom is satisfied. If two formal charges −1 and +1 are together then you can combine them to form a single lone pair.
