Question
Question: The number of degrees of freedom for a rigid diatomic molecule is _ _ _ _ _ _ _. A. 3 B. 5 C. ...
The number of degrees of freedom for a rigid diatomic molecule is _ _ _ _ _ _ _.
A. 3
B. 5
C. 6
D. 7
Solution
Hint: Degrees of freedom of molecules are total independent motions or energies that the molecules can have. A rigid diatomic molecule acts like a thin rod lying on the x-axis. Calculate the number of translations and rotational motions it can have.
Complete step by step answer:
Let us first understand what is meant by degrees of freedom.
The term degree of freedom of the system, tells us about the number of free or independent motions of the system. In other words, the degree of freedom is the number equal to the types of independent motions that the system can have.
When we say that an atom or a molecule is in motion, we know that it possesses some energy. The total energy of the atom or molecule is divided into different types. The total number of types (or forms) of energies possessed by the atom or the molecule is called the degree of freedom.
The independent motions can be translation and rotational or we can also say that a molecule possesses translation and rotational energies.
Consider a diatomic molecule. A diatomic molecule consists of two atoms separated by some distance as shown in the figure. These two atoms are attracted by strong forces and hence, they act as a linear rigid body.
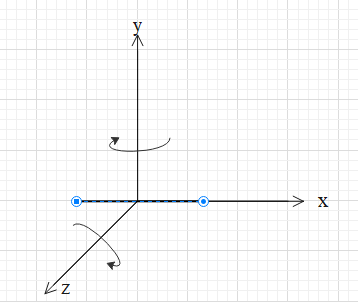
The molecule will have translational motions in all three directions. That is in the direction of the x-axis, y-axis and z-axis. Therefore, it has three independent translation energies.
The molecules will have two independent rotational motions. If the molecule lies on the x-axis, then one rotational motion is about y-axis (in the x-z plane) and the other one is about z-axis (in the x-y plane).
Therefore, a diatomic molecule has 5 degrees of freedom- 3 translational and 2 translational.
Hence, the correct option is B.
Note: The molecules of monatomic gas have 3 degrees of freedom. That is 3 translational.
The molecules of triatomic gas may have 5 or 6 degrees of freedom.
If the molecule is linear, then it has 5 degrees of freedom- 3 translational and 2 translational.
If the molecule is non-linear, then it has 6 degrees of freedom- 3 translational and 3 translational.
