Question
Question: The number of chain isomers possible for hydrocarbon \[{{C}_{5}}{{H}_{12}}\]is: A. 3 B. 5 C. 4...
The number of chain isomers possible for hydrocarbon C5H12is:
A. 3
B. 5
C. 4
D. 6
Solution
The word isomer is derived from two Greek words called iso + mer where iso means equal and mer means parts i.e. equal parts. Isomers are defined as those compounds which have the same chemical formula but different arrangement of molecules and the phenomenon of isomers is known as isomerism.
Complete step by step solution: Isomers are generally of two types: Structural isomerism and Geometrical isomerism. Structural isomers are also known by the name constitutional isomerism and it is of five types:
1. Chain isomerism: In this isomers are different in branching of chains.
2. Position isomers: Isomers differ with the arrangement of attachment of functional groups to different carbon atoms.
3. Functional isomerism: Those which have the same chemical formula but different functional groups.
4. Metamerism: Due to presence of different alkyl chains on each side of functional groups.
5. Tautomerism: Which differ in the position of proton and electron.
There are three possible chain isomers present for hydrocarbon C5H12which can be shown as follows:
1. n-pentane
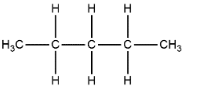
2. Iso-pentane or n-methyl butane
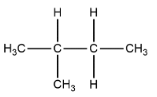
3. neo-pentane or 2,2-dimethylpentane
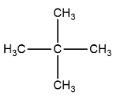
Hence from this we can conclude that there are 3 chain isomers present, option A is the correct answer.
Note: In constitutional type of isomerism the functional group and the atoms in the molecules of these isomers are linked in different ways. Whereas in geometrical isomerism compounds have the same chemical formula but different orientations of the atoms in three dimensional space.
