Question
Question: The number of \(C - H\) bonds in ethane \(({C_2}{H_6})\) molecule is: A.) Four B.) Six C.) Eig...
The number of C−H bonds in ethane (C2H6) molecule is:
A.) Four
B.) Six
C.) Eight
D.) Ten
Solution
The ethane molecule is an alkane in which all bonds are single bonds whether it is carbon-carbon bond or carbon-hydrogen bond. There is one carbon-carbon bond in ethane and remaining bonds are carbon-hydrogen bonds in it.
Complete answer:
In the given question, the molecule (C2H6) is called ethane. As we know that an alkane has general formula as CnH2n+2 and in given molecule by putting n=2, we get the ethane that is given as (C2H6). Hence, it is an alkene. Also, we know that in alkane molecules all the bonds are connected by a single bond. That is the carbon-carbon is connected with a single bond and also carbon-hydrogen is connected with a single bond. As we know that there is no hydrogen-hydrogen bond present in alkane.
Therefore, in ethane there is one carbon-carbon single bond combining two carbons and three hydrogens are attached with one carbon and remaining three hydrogens are connected with other carbon. The ethane molecule can also be written as CH3−CH3. The structure of the ethane molecule can be represented as:
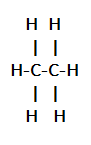
As we can see here, there is one carbon-carbon bond and six carbon-hydrogen bonds that are C−H bonds in thane molecules.
Hence, option B.) is the correct option.
Note:
Always remember that an alkane has the general formula as CnH2n+2 and it contains all single bonds, the alkene has general formula as CnH2n and it contains carbon-carbon double bonds and the alkynes has the general formula as CnH2n−2 and there is carbon-carbon triple bond.
