Question
Question: The number of bridging CO ligands present in [Co₂(CO)₈] and [Mn₂(CO)₁₀], respectively are...
The number of bridging CO ligands present in [Co₂(CO)₈] and [Mn₂(CO)₁₀], respectively are
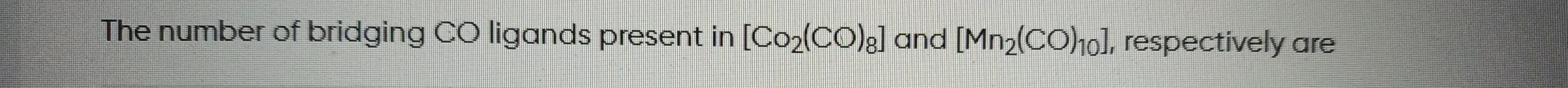
A
2 and 0
B
0 and 2
C
1 and 1
D
2 and 2
Answer
2 and 0
Explanation
Solution
The structure of Co2(CO)8 features two bridging CO ligands and a Co-Co bond. Each cobalt atom is also bonded to three terminal CO ligands. In contrast, Mn2(CO)10 has a Mn-Mn single bond and ten terminal CO ligands, with no bridging CO ligands.
