Question
Question: The number of bridging \(CO\) ligand (s) and \(Co - Co\) bond (s) in \(C{o_2}{(CO)_8}\) , respectiv...
The number of bridging CO ligand (s) and Co−Co bond (s) in Co2(CO)8 , respectively are:
a.) 0 and 2
b.) 2 and 0
c.) 4 and 0
d.) 2 and 1
Solution
Spanning ligands can be fundamentally classified as ligands that are associating or joining or at least two iotas/atoms together. These ligands are generally used to interface metal particles. Presently, these ligands may either be mono - nuclear or polyatomic in nature.
Complete step by step answer:
As can be seen from the structure of Co2(CO)8, there are two CO crossing over ligands and one Co−Co (Metal-Metal) bond.
Number of M−M Bonds = 218×n−VE
n = number of metals
(i) Co2(CO)8
VE=9×2+2×8
⇒VE=34
Number of M−M Bonds = 218×2−34 = 1
So the number of Co−Co bond is 1.
Verification:
Co2(CO)8 is Octahedral in nature
So, the number of ligands in Co2(CO)8 are 6.
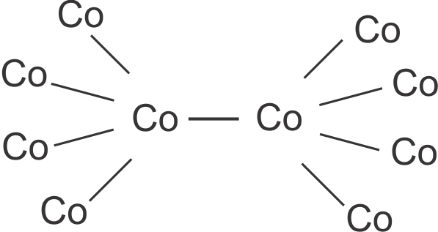
So, if we re draw the above diagram we would get 2 bridging CO ligands in Co2(CO)8
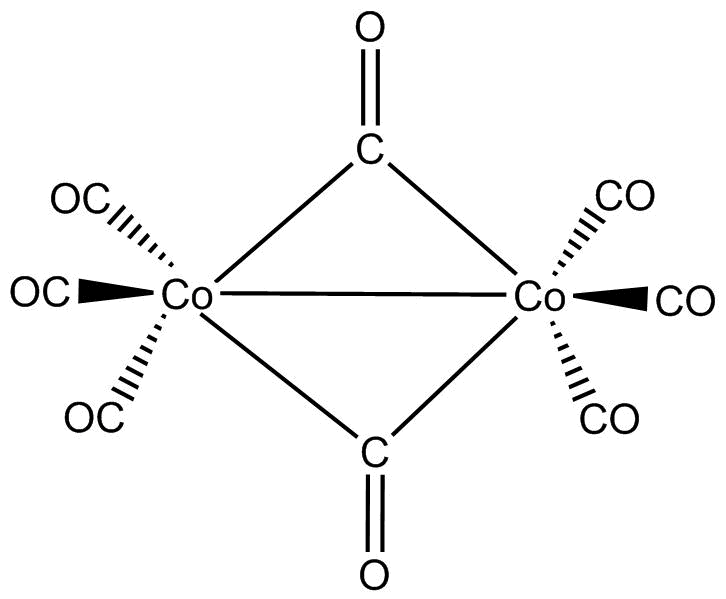
The correct answer is option “D” .
Additional Information :
Bridging ligands: A bridging ligand is a ligand that interfaces at least two molecules, normally metal particles. The ligand might be nuclear or polyatomic. Essentially all intricate natural mixes can fill in as bridging ligands, so the term is normally limited to little ligands, for example, pseudo-halides or to ligands that are explicitly intended to connect two metals.
Note: In naming a complex wherein a solitary particle spans two metals, the connecting ligand is gone before by the Greek letter mu, μ, with a superscript number indicating the quantity of metals bound to the crossing over the ligand. μ2 is frequently signified basically as μ. While depicting coordination buildings care ought to be taken not to mistake μ for η, which identifies with hapticity. Ligands that are not connecting are called terminal ligands.
