Question
Question: The number of atoms in the cycle structure of D-fructose is A. \[5\] B. \[6\] C. \[4\] D. \[...
The number of atoms in the cycle structure of D-fructose is
A. 5
B. 6
C. 4
D. 7
Solution
We know that the sugars can be written using Haworth's formula. Pyranose and Furanose are the oxides rings that resemble the oxygen-containing heterocyclic ring pyran and furan respectively. These are five or six-membered rings where one of the positions is occupied by oxide.
Complete answer:
Fructose occurs in fruits and it is called the fruit sugar. It is also present in honey and sweet fruits along with glucose. In the combined state, it is also present in disaccharide and polysaccharide (insulin). Its molecular formula is C6H12O6. It contains the keto group at C−2and the six carbon atoms are arranged in a straight chain. The Fischer projection of the fructose can be converted into the cyclic structure. Like glucose, fructose has a cyclic structure. The hemiacetal is formed by the intermolecular combination of, keto group and −OH a group of C6 the atom. As a result, C2 the atom becomes asymmetric and therefore D-fructose has two possible isomers.
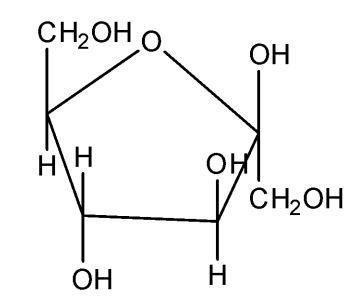
Therefore, the correct answer is option (A).
Additional Information: Pyranose rings are formed by the reaction of the keto group on the carbon number two of sugar with the hydroxyl which is at the carbon one. This formation goes through the hemiacetal. This structure is similar to the furan ring. Thus here we know that the five-membered rings of oxide are called the pyranose. Due to the formation of an oxide ring, the new asymmetric carbon atom is created at the carbonyl carbon, which is called an anomeric carbon atom. Two different configurations are possible at the anomeric carbon atom and they are called the anomers.
Note:
Remember that when the bond in a chemical compound is broken, then there is a need for energy hence the bond energy will have a positive value. And when the bond in a chemical compound is formed, then the energy will be released hence the bond energy will have a negative value.
