Question
Question: The number of acyclic structural isomers represented by molecular formula \[C{}_4H{}_{10}O\] is: A...
The number of acyclic structural isomers represented by molecular formula C4H10O is:
A. 7
B. 6
C. 8
D. 5
Solution
A structural isomer of a compound can be understood as a compound where the isomeric compound has the same number of atoms of each element as that of the original compound, but the The molecular structure of the isomeric compound is different. To put it in simpler terms, structural isomerism can be understood as the phenomenon when the two or more compounds have the same number of atoms of each constituent element, but their molecular structures are different.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some
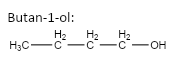
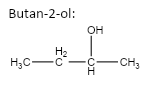
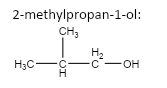
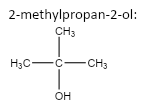
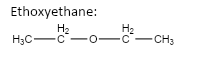
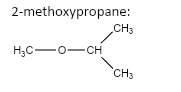
important basic concepts.Now, this means that the orientation of the atoms has many possible configurations. These configurations include both straight chain compounds as well as cyclic compounds. The term acyclic structural isomers refer to all the possible isomeric structures except the cyclic molecular structures.Now, these isomers can be formed by the rearrangement of the given atoms into different functional groups as well. Such isomers are identified as functional group isomers.Now, moving to the question, the compound that has been given to us has the molecular formula C4H10O . Hence, the acyclic structural isomers for this compound can be given as:
1.
2.
3.
4.
5.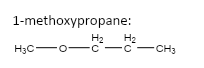
6.
7.
Hence, Option A is the correct option
Note: Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism,in which the atoms and bonding scheme are the same, but only the relative spatial arrangement of the atoms are different.
