Question
Question: The number of 1p-bp repulsions present in \(\text{ Cl}{{\text{F}}_{\text{3}}}\text{ }\) at nearly 90...
The number of 1p-bp repulsions present in ClF3 at nearly 90-degree angle are:
A) 1
B) 2
C) 3
D) 4
Solution
The ClF3 has trigonal bipyramidal structure. There are a total of 3 bond pairs and two lone pairs. The two lone pairs are at the equatorial position. Such that the structure experiences the minimum repulsion. The repulsion between the electrons pairs is as shown below,
bp−bp < bp−lp <lp−lp
Complete step by step answer:
The chlorine trifluoride ClF3 is an interhalogen compound. It is a XX3′ type of compound, where X is the chlorine atom and X !!′!! is the fluorine atoms surrounding the central chlorine atoms.
The chlorine is a central atom. It has 7 valence electrons. The electronic configuration of chlorine is as flows,
Cl = [Ar]3s2 3p5
The valence s, p, and d orbitals take part in the hybridisation. It results in the formation of 5 hybrid orbitals. Thus the hybridization of chlorine is sp3d .
The ClF3 has a trigonal bipyramidal geometry. It has three bond pairs and the valence electrons on the chlorine are acted as the two other substituents on the chlorine atom.
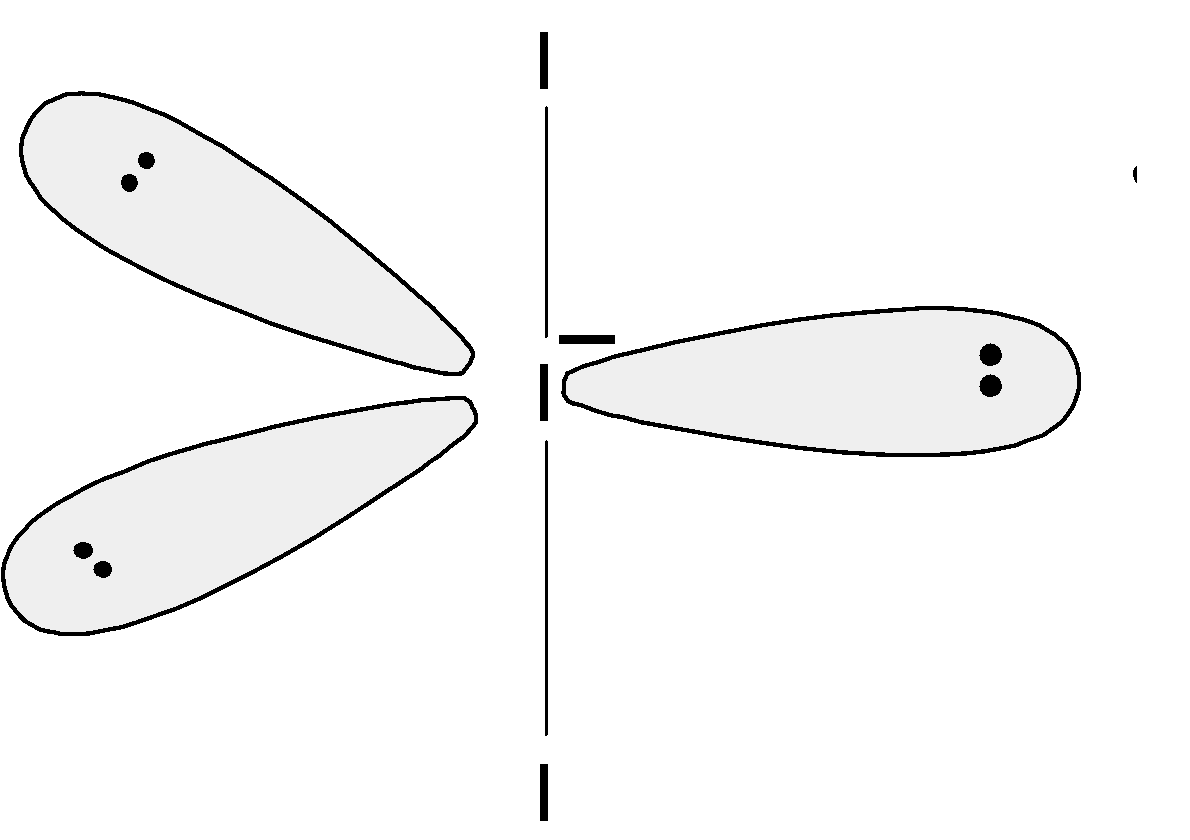
The shape of the ClF3 is determined by considering the position of the lone pairs and the bond pairs. Even though we know there are three bonding pairs and two lone pairs, the biggest question is where is to place the lone pairs. The structures adopt the configuration in which the electrons experience the minimum interaction. There are three possibilities to place the lone pairs in the ClF3 molecules are follows:

Let’s first consider the structure (I), the lone pairs occupy the angular spaces and are located closer to the atoms than the bond pairs. Thus, the interaction between the lone pairs at the angle is found to be greater than the bonding pair.
In the structure (II), the lone pairs are located in the axial and one in the equatorial plane. The bonding pairs experience the bond pair-bond pair, bond pair-lone pair, and lone pair –lone pair interactions.
In the (III) structure, the two lone pairs are at the 1800 angles. There are a total of 6 lone pair and bond pair interactions.
Let's tabulate the structure and the angles. We have,
| Structure (I)| Structure (II)| Structure (III)
---|---|---|---
900 | 2 bp−bp 4 bp−lp | 2 bp−bp 3 bp−lp 1 lp−lp| 6 lp−bp
Here bp stands for the bonding pair and lp stands for the lone pair.
From this table, when the 2 lone pairs are placed at the equatorial position they do not contain the lone pair –lone pair interaction. Thus the ClF3 has the trigonal bipyramidal structure. The equatorial lone pairs repels each other. These lone pairs further pushes the bond pair. The structure has a distorted trigonal bipyramidal structure. This is also known as the T –shaped geometry.
The structure of ClF3 the molecule is,
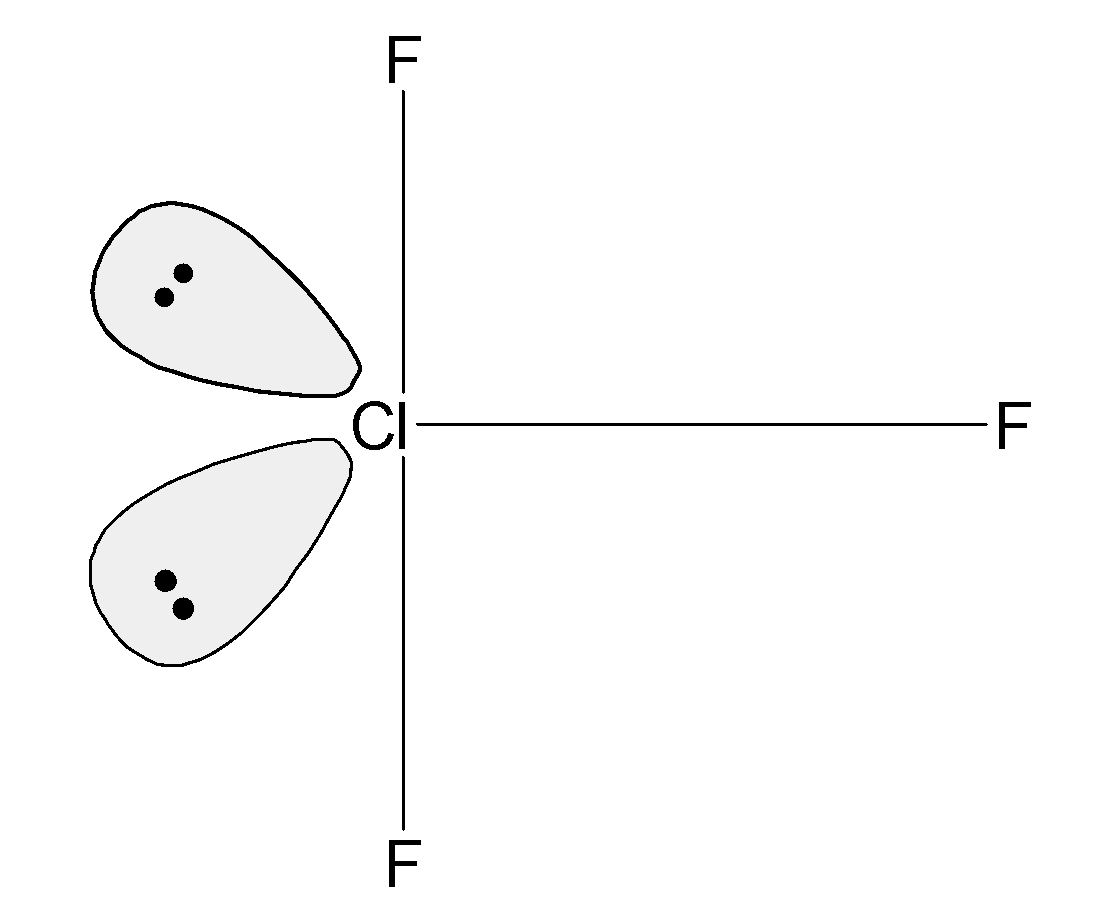
The total lone pair and bond pair repulsion at the 900 are equal to the 4. The equatorial lone pair interact with the adjacent bond pair ( Cl−F) in the angular plane and the two lone pairs interact with the axial bond pairs. Thus there are a total of 4 interactions between the bond pair and lone pair.
So, the correct answer is “Option D”.
Note: Note that, using the same explanation we can show that in trigonal bipyramidal geometry the electrons pairs or lone pairs are situated best at the equatorial places. For example, in I3− . The structure of the I3− is as shown below: