Question
Question: The non-aromatic compound among the following is:- A 
B
C
D
Solution
Non-aromatic compounds are cyclic compounds which do not have a closed loop of p-orbitals that is because it is not conjugation of π (pi) – electrons. Also, the non-aromatic compounds are non- planar ring systems.
Complete step by step answer:
A: As we know that cyclopentadiene (C5H6) is not having conjugation so definitely it is not an aromatic compound. Also, the huckel's rule (4n+2) electrons conjugation which is applicable for aromatic compounds, is not being followed here.
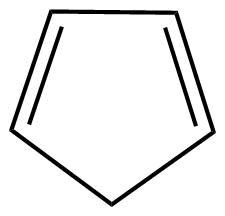
Hence, we can say that this is a non-aromatic compound.
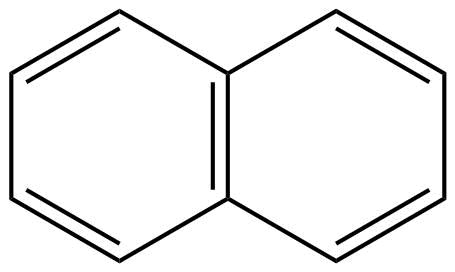
B: Naphthalene is an aromatic compound because it shows the continuous delocalisation of pi (π) – electrons that are naphthalene have conjugation.
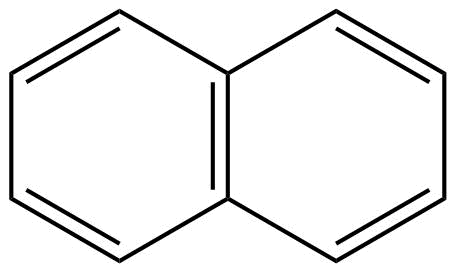
Moreover, naphthalene is also having a planar structure. Hence, we can say that naphthalene is an aromatic compound.
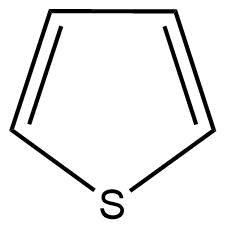
C: Thiophene is a heterocyclic compound with the chemical formula C4H4S. It is a planar five-membered ring.
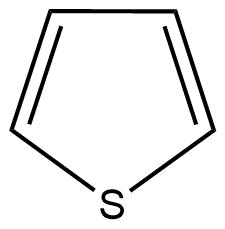
Here, the conjugation of delocalised π (pi) electrons can be also seen. Hence, thiophene is also an aromatic compound.
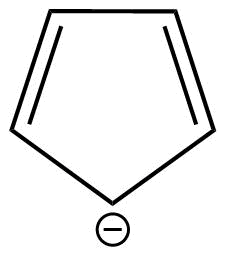
D: Cyclopentadienide is a monocyclic aromatic compound.
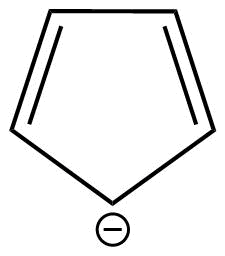
This is because cyclopentadienide shows conjugation and huckel's rule is also applicable in case of this compound.
So, the correct option is (A).
Note:
The non-aromatic compounds without a ring structure are termed without a ring structure are termed as aliphatic whereas those with a cyclic structure are called alicyclic.
