Question
Question: The net work done through a series of changes reported in the figure at the end of the cycle for an ...
The net work done through a series of changes reported in the figure at the end of the cycle for an ideal gas is equal to:
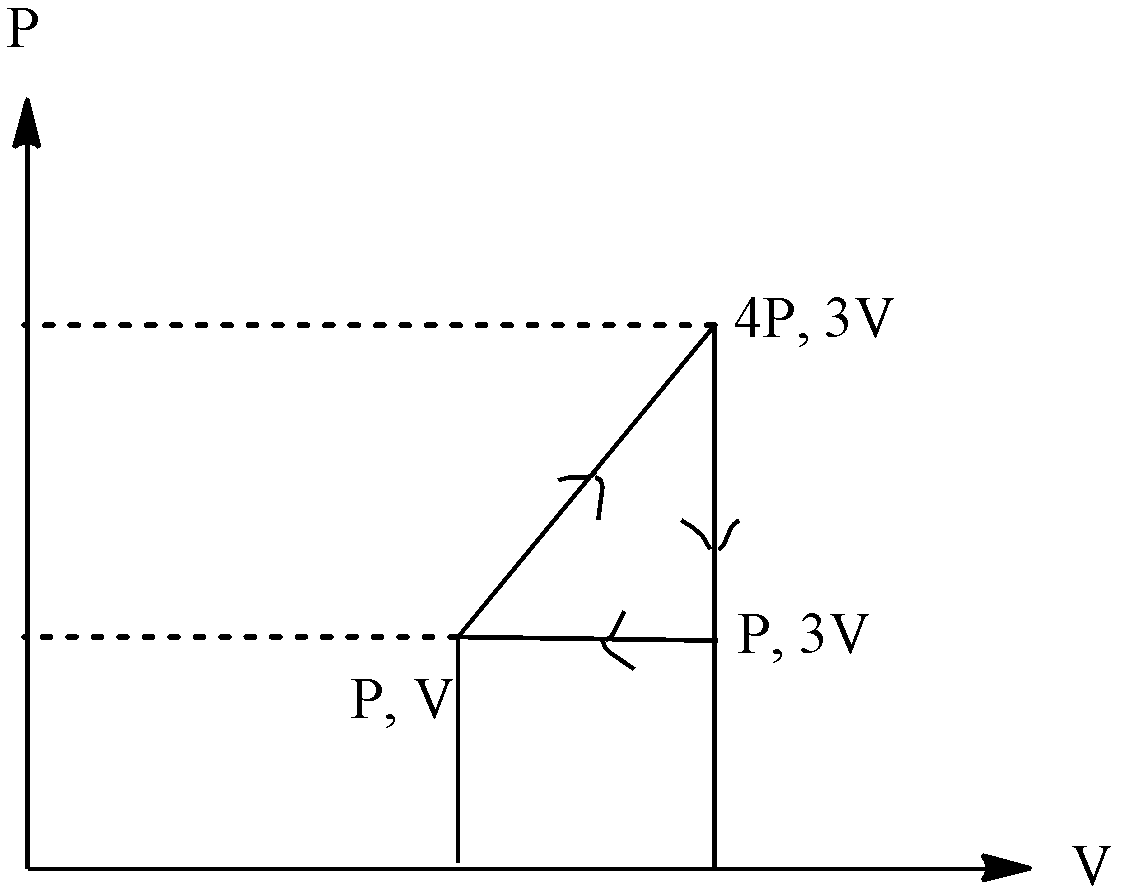
(a) 0
(b) -2 PV
(c) -3 PV
(d) -5 PV
Solution
The work done of the system is calculated by taking the product of pressure and change in volume in the system, but when the graph is given then we have to find the area in which the work is occurring. In the graph given above the work is done in a triangle shape, so we can calculate the work done by taking the product of the height of the triangle and the length of the base of the triangle and divide it by 2.
Complete answer:
There are many parameters that are used to define the work done in the system like, pressure, volume, etc. The work done of the system is calculated by taking the product of pressure and change in volume in the system. But the value of pressure and volume is not given, instead the graph is given then we can calculate the area in the graph to calculate the work done.
In the graph given above the work is done in a triangle shape, so we can calculate the work done by taking the product of the height of the triangle and the length of the base of the triangle and divide it by 2.
Work done=21 x height x base
The graph is:
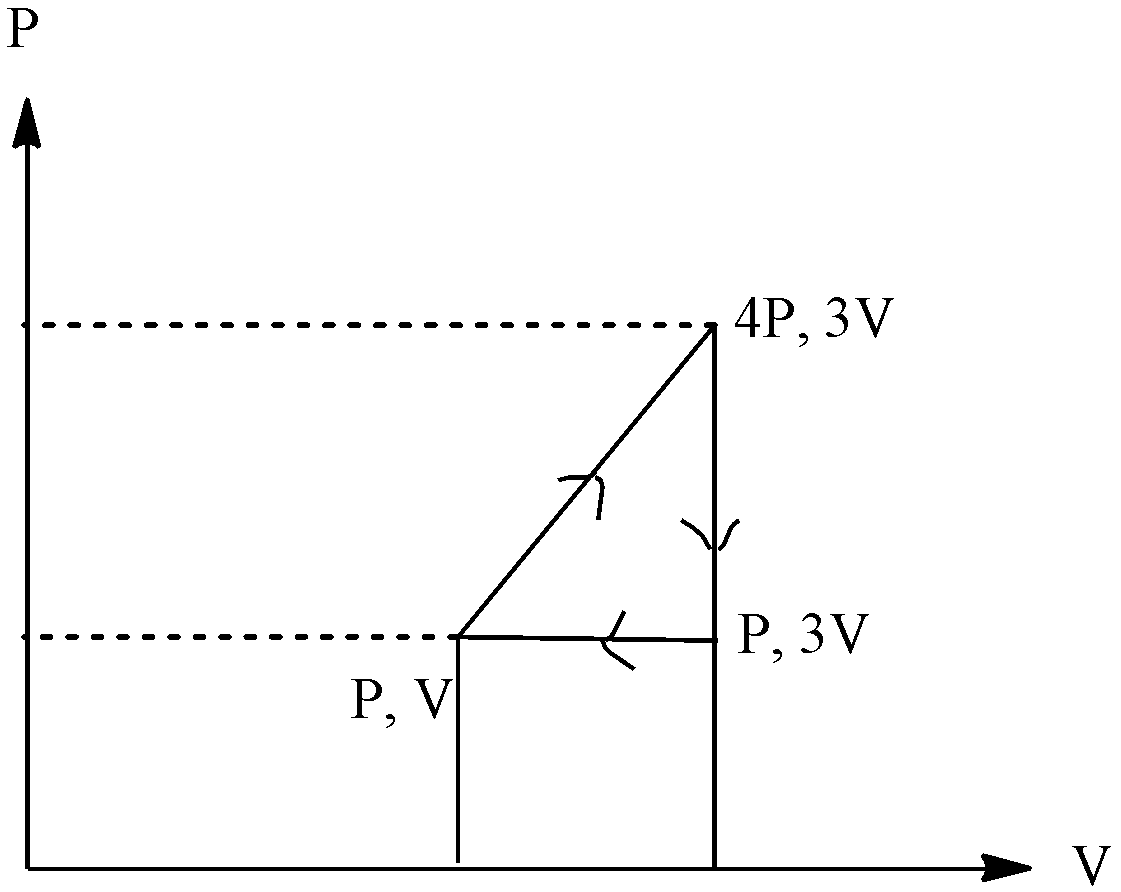
The value of height will be the change in the pressure, we can see that pressure changes from 4P to P, and the length of the base changes in volume, the volume changes from 3V to V. since work is done by the system, the value will be negative, putting the values, we get:
Work done=−21 !![!! (4P-P) x (3V-V) !!]!!
Work done=−21 x 6PV
Work done=−3PV
Therefore, the work done is -3PV.
So, the correct answer is “Option (b)”.
Note:
There are sign conventions that must be taken care of when we calculate the work done, i.e., if the work is done by the system then use the negative system and if the work is done on the system then use a positive sign.
