Question
Question: The most suitable reagent for the following conversion is: 
(A) H2,Pd/C,quinoline
(B) Zn/HCl
(C) Hg2+/H+,H2O
(D) Na/liquidNH3
Solution
In this given reaction, but - 2 - yne is converted to cis - 2 - butene , this is an example of conversion of alkynes to cis/trans alkenes with the help of suitable reagents. This process is called birch reduction of alkynes
Complete Step-by-step Answer:
Alkynes are the hydrocarbons that have a general formula of {C_n}{H_{2n - 2}} . In alkynes there is a triple bond between two carbon atoms. They are unsaturated and non-polar hydrocarbons. They generally dissolve in Organic solvents but are non-soluble in water. The first member of the homologous series of alkynes is Acetylene or Ethyne.
In the question we are given that but - 2 - yne is converted to cis - 2 - butene , this conversion is an example of birch reduction.
In birch reduction, Alkynes are reduced to cis/trans alkenes with the help of Na/liquidNH3 and H2,Pd/C,quinoline . Na/liquidNH3 is used to form trans alkene and H2,Pd/C,quinoline is used to form cis-alkene. H2,Pd/C,quinoline is a hydrogenation catalyst. It is used for the hydrogenation of alkynes to convert them into alkenes by breaking the triple bond into double bonds. H2,Pd/C,quinoline is called lindlar’s catalyst. It is a poisonous catalyst which is used for the partial hydrogenation of alkynes. The quinolone present in the reagent stops the complete hydrogenation of alkynes and prevents the formation of alkanes. The lindlar’s catalyst has three components. The conversion of but - 2 - yne to cis - 2 - butene is given as:
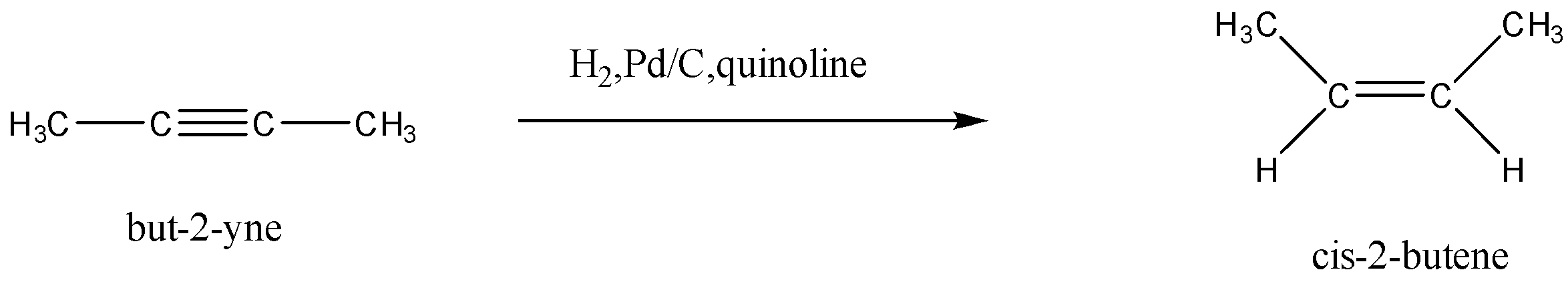
Hence the reagent used is H2,Pd/C,quinoline .
Therefore, option (A) is correct.
Note:
Cis and trans alkenes are known as geometric isomers. They are the examples of stereoisomers in chemistry. In this isomerism the pairs of molecules have the same molecular formula but the functional groups have a different orientation in three-dimensional space.
