Question
Question: The most stable conformer of Cis-1,4-cyclohexan-1,4-diol is: A.Diaxial boat form B.Diequatorial ...
The most stable conformer of Cis-1,4-cyclohexan-1,4-diol is:
A.Diaxial boat form
B.Diequatorial boat form
C.Diaxial chair form
D.Diequatorial chair form
Solution
Draw all the forms one by one and compare their stability. Also check the possibility of 1,3 diaxial repulsion and steric hindrance while assigning the stability priority.
Complete step by step answer:
We will draw the structure for every form and then we will decide which is stable or which is not. Let’s start with option A-Diaxial boat form
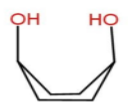
Here we can see there will be a strong intramolecular hydrogen bonding. In cyclohexane boat form 1, 4 interaction is known as Flagpole interaction which makes it unstable. But in case of Cis-1,4-cyclohexan-1,4-diol ,−OH groups are present instead of hydrogen then there will be a strong hydrogen bonding and no flagpole interaction. Hence Diaxial boat form is stable.
Let’s move to option B – Diequatorial boat form.
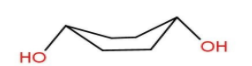
Here we can see that −OH groups are at equilibrium but hydrogens present at axial position will show flagpole interaction. Due to this interaction Diequatorial boat form is unstable.
Now Option C- Diaxial chair form.
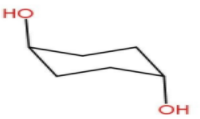
In Diaxial chair form there is a high possibility of 1, 3-Diaxial repulsion . Due to this interaction there will
be a strong steric hindrance in this form. Hence this is also unstable.
Let’s move to the last option D- Diequatorial form.
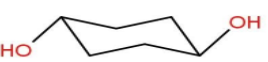
Though −OH groups being bulky are placed at equatorial positions. Still there is a 1,3-Diaxial repulsion present in this form hence, making it unstable.
So, we have seen from all the four forms Diaxial boat form is the most stable form.
Hence the correct answer is option A.
Note:
Not every time equatorial forms will be the most stable one. There can be exceptions where axial and boat forms can be more stable than equatorial and chair forms. Take the example of 3-aminocyclohexanol. You might think that equatorial form must be a stable one because −NH2 is bulky than hydrogens. So if we keep the bulkier group at equatorial position it will provide stability to the compound. But that’s not the case. Here, also in axial form strong intramolecular hydrogen bonding takes place making the axial form more stable than equatorial form
