Question
Question: The most stable conformation of \[cis - 1,4 - di - t - butyl{\text{ }}cyclohexane\] is? A. Boat ...
The most stable conformation of cis−1,4−di−t−butyl cyclohexane is?
A. Boat
B. Chair
C. Twist boat
D. Half chair
Solution
You can make the different conformation by taking bulky groups of opposite sides. It was seen that bulky groups always approach through axial position. So, we always see the chair confirmation that we get an idea about the two attacking sites, equatorial and axial position. But many cases are there where twist boats are seen.
Complete step-by-step answer: Cyclohexane is a type of hydrocarbon which can convert in four types of arrangement to see the most stable configuration. There are bulky groups which hindered the position and thus repel other groups, due to the steric hindrance it is possible to make a valid structure in which the position of bulky groups are away from each other.
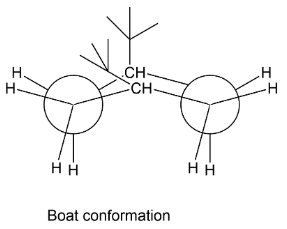
We have given four types of arrangements and we have to tell that which is most suitable among all these so that the compound cis−1,4−di−t−butyl cyclohexane will have a steric free arrangement. Let’s see first the boat form of cis−1,4−di−t−butyl cyclohexane in this the two bulky tertiary butyl groups are just face to face by which steric hindrance make the arrangement less stable thus this is not stable conformation.
In the chair form the conformation is staggered it means the hydrogen are not face to face arranged but they are arranged in opposite positions. As if we see the figure below, the hydrogens are just opposite to each other making staggered form and bulky groups are axially arranged so we have structure like this,
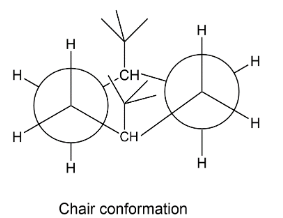
Here also there is hindrance between two bulky tertiary butyl groups because they are both arranged axially. It is also not a stable conformation.
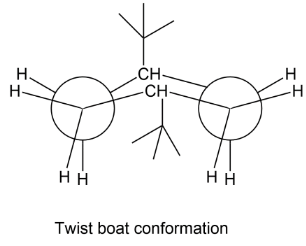
Next we have a twist boat, where one bond is twisted so that one tertiary butyl group will be arranged just opposite to that of the first group as in the first structure of boast form. This is called twist boat conformation. Here there is no steric hindrance hence it is our answer.
Also let’s see the last option is the half chair, in half chair conformation five carbons come in the same plane thus a much more amount of steric hindrance produced hence it is also a less stable conformation.
Therefore, option C is correct.
Note: While solving the conformation types of question of any substrate, try to make the group which are bulky at axial positions. If the group is just face to face then you can make one group above and another group lower. By this way the conformation will become less hindered. There are some groups like hydrogen, deuterium and fluorine which are not bulky so their interaction with other groups is less.
