Question
Question: The most stable carbonium ion is...
The most stable carbonium ion is
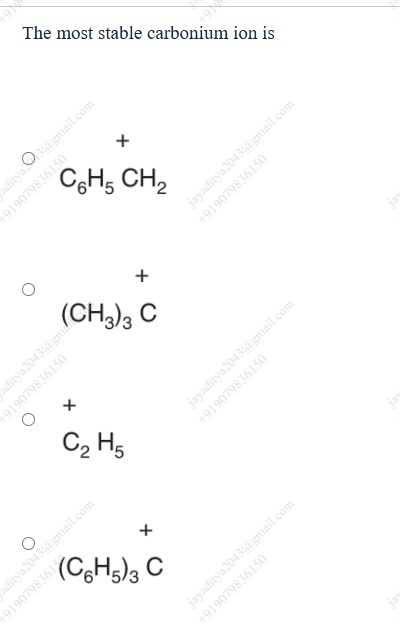
A
+C6H5 CH2
B
+ (CH3)3 C
C
+C2 H5
D
+ (C6H5)3 C
Answer
+ (C6H5)3 C
Explanation
Solution
The stability of carbocations is influenced by inductive effects, hyperconjugation, and resonance. Resonance stabilization is generally the most significant factor, especially when it leads to extensive delocalization of the positive charge.
Let's analyze each option:
- +C6H5 CH2 (Benzyl carbocation): This is a primary carbocation, but it is stabilized by resonance with one phenyl ring. The positive charge can be delocalized onto the ortho and para positions of the benzene ring.
- + (CH3)3 C (tert-Butyl carbocation): This is a tertiary alkyl carbocation. It is stabilized by the positive inductive effect (+I) of the three methyl groups and by hyperconjugation involving the nine α-hydrogens.
- +C2 H5 (Ethyl carbocation): This is a primary alkyl carbocation. It is stabilized by the positive inductive effect (+I) of the methyl group and by hyperconjugation involving the three α-hydrogens. Primary carbocations are generally less stable than secondary and tertiary alkyl carbocations.
- + (C6H5)3 C (Triphenylmethyl carbocation): This carbocation has a central carbon atom with a positive charge bonded to three phenyl rings. The positive charge can be extensively delocalized into all three phenyl rings through resonance. This provides a very high degree of resonance stabilization.
Comparing the stability factors:
- Alkyl carbocations stability order: Primary < Secondary < Tertiary. Ethyl (1∘) < tert-Butyl (3∘).
- Resonance stabilization is generally more effective than hyperconjugation and inductive effects in simple alkyl systems. Benzyl carbocation (resonance with 1 ring) is more stable than tert-Butyl carbocation (3∘ alkyl).
- The extent of resonance stabilization increases with the number of attached phenyl rings. Triphenylmethyl carbocation (resonance with 3 rings) is significantly more stable than Benzyl carbocation (resonance with 1 ring).
Therefore, the stability order among the given options is:
Ethyl (1∘ alkyl) < tert-Butyl (3∘ alkyl) < Benzyl (1 phenyl resonance) < Triphenylmethyl (3 phenyl resonance).
The triphenylmethyl carbocation (+ (C6H5)3 C) has the most extensive resonance stabilization and is therefore the most stable among the given options.
