Question
Question: The most stable carbocation is....
The most stable carbocation is.
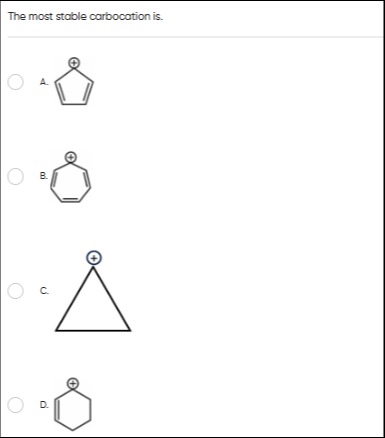
A
Cyclopentadienyl cation
B
Cycloheptatrienyl cation
C
Cyclopropyl cation
D
Cyclohexenyl cation
Answer
Cycloheptatrienyl cation
Explanation
Solution
Carbocation stability is governed by resonance and aromaticity. The cycloheptatrienyl cation (B) is aromatic (6 pi electrons, cyclic, planar, conjugated), hence it is the most stable. The cyclohexenyl cation (D) is resonance stabilized. The cyclopentadienyl cation (A) is not aromatic. The cyclopropyl cation (C) is strained.
