Question
Question: The most stable carbocation among the following is: A.
B.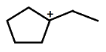
C.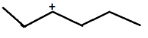
D.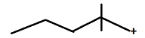
Solution
Carbocations are the electron deficient species because they have incomplete octet.
We know that Stability of carbocations depends on three factors :
(1)Resonance Effect (2) Hyperconjugation (3)Inductive effect
Complete step-by-step answer: As we all know that formation of carbocation takes place when there is heterolytic fission and results in incomplete octet. They are also known as electron deficient species. So the stability of carbocation increases as the number of electron donating groups attached to it increases.
That’s why the stability order of carbocation is : 3o>2o>1o.
So, to check the stability of carbocation we have to consider any of the three effects i.e Resonance(+R),Hyperconjugation(+H) or Inductive effect (+I).
In this question , with Inductive effect there is also Hyperconjugation effect. HENCE, Hyperconjugation effect dominates over Inductive effect . And the Hyperconjugation effect is stronger than the Inductive effect.
IN OPTION (A)
C⊕ atom is surrounded by 4 α−Hatom.
IN OPTION (B)
C⊕ atom is surrounded by 6 α−Hatom.
IN OPTION ( C )
C⊕ atom is surrounded by 4 α−Hatom
IN OPTION (D)
C⊕ atom is surrounded by 0 α−H atom
As we all know , more the number of α−H atoms, more is the stability of carbocation. So we can conclude that the number of α−H atoms is directly proportional to the stability of carbocation.
So by this we can conclude that the correct answer of the above question is OPTION (B).
Where the number of α−H Atom is higher than other options.
Note: ALWAYS REMEMBER : +R>+H>+I Rule
Resonance dominates Hyperconjugation while Hyperconjugation dominates Inductive Effect.
So we have to check the stability of Carbocation according to this rule.
