Question
Question: The most reactive towards \(S{N_1}\) is : ( A ) \(PhC{H_2}Cl\) ( B ) \(PhCl\) ( C )\(PhCHCl(C...
The most reactive towards SN1 is :
( A ) PhCH2Cl
( B ) PhCl
( C )PhCHCl(CH3)
( D )P−NO2C6H4CH2Cl
Solution
Order of reactivity towards SN1 reaction depends on the formation of intermediate carbocation and another factor is degree of carbocation formed.
Complete step-by-step answer: SN1reaction is the nucleophilic substitution reaction . They are the unimolecular reaction because the rate of SN1 reaction depends only on the concentration of one reactant.
Order of reactivity of SN1 nucleophilic substitution reaction depends on the degree of carbocation i.e; 3∘>2∘>1∘.
Let's check the above given options :
( A ) PhCH2Cl → 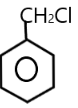
The carbocation of this structure is 1∘ . As we know that 1∘ carbocation is less reactive towards SN1 nucleophilic reaction .
( B ) PhCl → 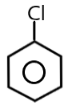
The carbocation of this structure is 2∘. So as we know that 2∘ carbocation is more reactive towards SN1 nucleophilic reaction than the 1∘ carbocation .
( C ) PhCHCl(CH3) →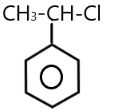
The carbocation of this structure is 2∘ . So 2∘ carbocation is more reactive towards SN1 nucleophilic reaction than the 1∘ carbocation . The presence of methyl −CH3 group also increases the positive charge present on the central carbon atom .
( D ) p−NO2C6H4CH2Cl → 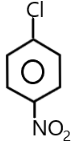
The carbocation of this structure is 1∘. As we know that 1∘ carbocation is least reactive towards SN1 nucleophilic substitution reaction . The presence of an electron withdrawing group at the para position of the phenyl group also decreases the positive charge on carbocation .
So, from the above explanations we can conclude that the CORRECT answer of the above question is OPTION ( C ) .
Note: We have to remember that the order of reactivity of SN1 nucleophilic substitution reaction depends on the degree of carbocation . Also presence of electron withdrawing group and electron donating group contributes in the reactivity towards SN1 nucleophilic substitution reaction .
