Question
Question: The most direct malonic ester synthesis of the 3 – phenyl propanoic acid would involve the use of: ...
The most direct malonic ester synthesis of the 3 – phenyl propanoic acid would involve the use of:
A. C6H5CH2CH2CH2CI
B. C6H5CH2CH2CI
C. C6H5CH2CI
D. CICH2COOH
Solution
The reaction of benzyl chloride with diethyl malonate in ethoxide/ethanol produces the alkylated product and the acidification hydrolyses the esters and the intermediate diacid decarboxylates to form the final product i.e.3 – phenyl propanoic acid.
Complete step by step answer:
Malonic ester is a reagent which is specifically used in a reaction which converts alkyl halides to carboxylic acids called the Malonic Ester Synthesis. Malonic ester synthesis is a synthetic procedure used to convert a compound that has the general structural formula 1 into a carboxylic acid that has the general structural formula 2.
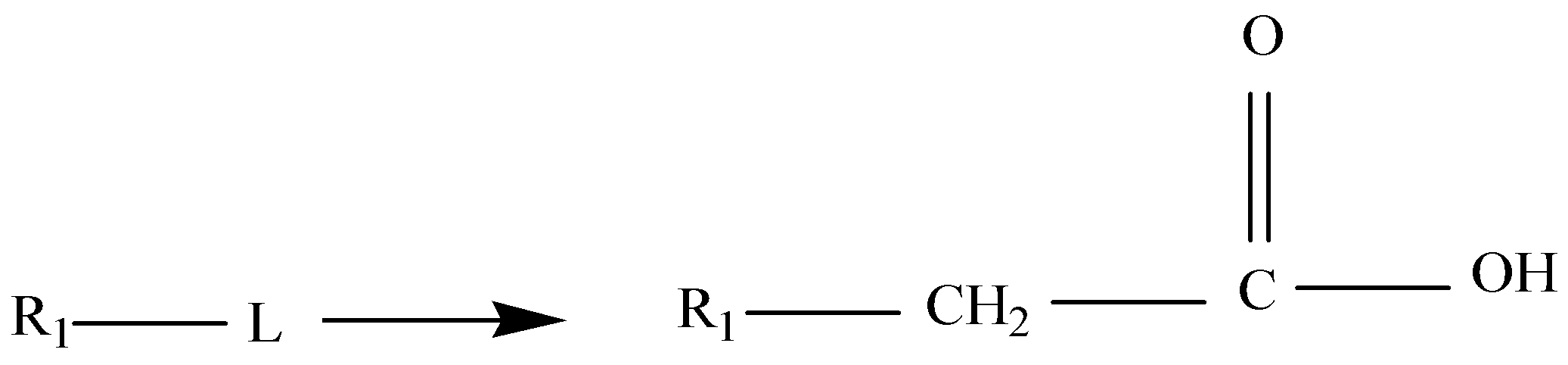
Where R1 is an alkyl group and L is a leaving group. The group −−CH2CO2H in 2 is contributed by a malonic ester, hence the term malonic ester synthesis.
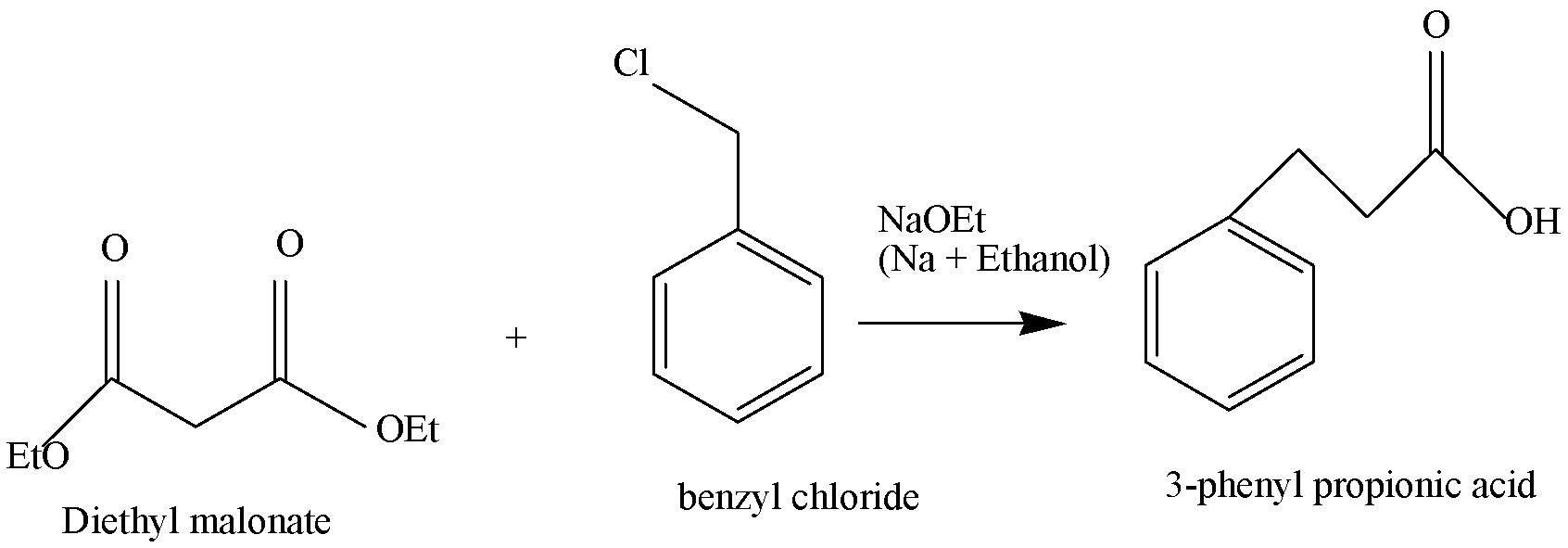
Firstly, freshly prepared sodium ethoxide reacts with diethyl malonate giving enolate ions which alkylate with the benzylated malonic ester. Reaction of benzyl chloride with diethyl malonate in ethoxide/ethanol yields the alkylated product. Acidification hydrolyses the esters and the intermediate diacid decarboxylates to form the final product, 3 – phenyl propanoic acid in the presence of a base like NaOH (in the above reaction).
So, the most direct malonic ester synthesis of the 3 – phenyl propanoic acid would involve the use of benzyl chloride i.e. C6H5CH2CI .
Therefore, the correct answer is option (C).
Note: One of the most valuable methods to prepare carboxylic acids makes use of ethyl malonate which is the malonic ester. 3 – phenyl propanoic acid is used as a flavouring agent, food additives, fragrance, spices etc. As it acts as a preservative.
