Question
Question: The monomers used in the preparation of dextron are: (A.) 3-hydroxy butanoic acid and 3-hydroxy pe...
The monomers used in the preparation of dextron are:
(A.) 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid
(B.) Aminocaproic acid and glycine
(C.) Isobutylene and isoprene
(D.) Lactic acid and glycolic acid
Solution
The monomers used in dextron are well-known acids. You can find one of them in the curd (one of the milk products). Now try to answer this question accordingly.
Complete Solution :
- We should know that to form a Polymer, Monomers are bonded to another molecule.
- Polymerization is the process of the formation of large molecules by the combination of a large number of small molecules.
- The compound Dextron was the first biodegradable suture made from biodegradable polyesters for the postoperative stitcher.
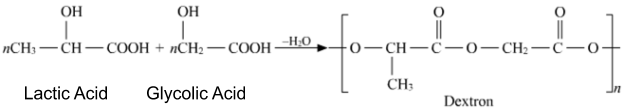
- The Monomers used in the preparation of Dextron are Glycolic acid and Lactic acid.
Mechanism of the reaction between the lactic acid and glycolic acid-
Step I-
The addition reaction between lactic acid and glycolic acid takes place.
Step II-
As lactic acid and glycolic acid are the same types of monomers thus, they are copolymers. Dextron is the product of copolymerisation of lactic acid and glycolic acid.
Step III-
Elimination of water molecules takes place while the formation of polymers.
This is shown as:
Additional information
- Biodegradable polymers are polymers which break down after its planned use. For example, PHBV, starch, cellulose, dextron, etc.
- Non-biodegradable polymers are polymers that don’t break down by natural processes over time. For example, polyethylene, polyvinyl chloride, nylon, Teflon, etc.
So, the correct answer is “Option D”.
Note: You should not confuse yourself with another compound that has also a similar name, Dextran. It is a complex branched glucan (polysaccharide derived from the condensation of glucose). The polymer main chain consists of α-1,6 glycosidic linkages between glucose monomers, with branches from α-1,3 linkages.
