Question
Question: The monomer used to produce orlon is: A. \(C{H_2} = CHF\) B. \(C{H_2} = CC{l_2}\) C. \(C{H_2} ...
The monomer used to produce orlon is:
A. CH2=CHF
B. CH2=CCl2
C. CH2=CHCl
D. CH2=CHCN
Solution
First we should be aware of the monomer which is used in making the polymer fabric of orlon. Now the monomer is the small of basic integrative constituents which are reacted by the process of polymerization for the making of polymers. Here the monomer is CH2=CHCN .
Complete answer:
Here in the given question statement the question is regarding the terminology of the type of the substance which is formed when two or more atoms unite by the use of any force. This on a much permanent basis.
Orlon is a Strong and warm acrylic fiber. It has multiple uses but most often is used for making the sweaters and tracksuits and in many cases as the linings for many kinds of boots and gloves. Apart from that they are also used in the furnishing fabrics and carpets. It is mostly manufactured in the form of a filament, then cut into short staple lengths similar to wool hairs, and spun into yarn.
Now we know that the monomer which is used to produce orlon is acrylonitrile (vinyl cyanide) CH2=CHCN
So we can say that Orlon is polyacrylonitrile and It is also an additional polymer.
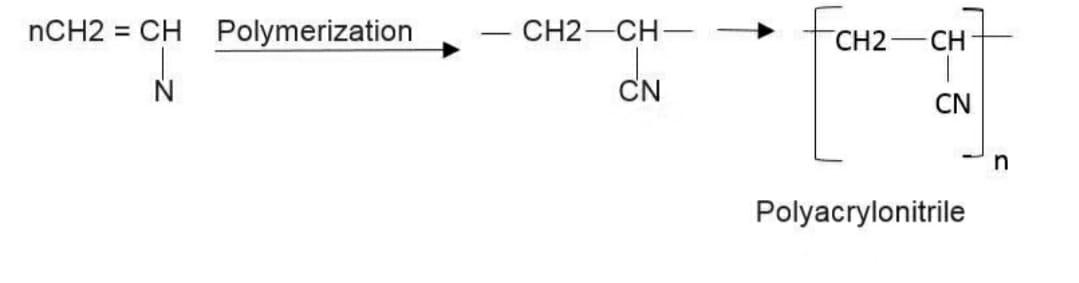
Here is a chemical reaction showing the formation of the polymer or the process of polymerization taking place. This is done under certain circumstances and specific times.
The chemical reaction is :
 Therefore the correct option is option D, Impure metal M.
Therefore the correct option is option D, Impure metal M.
Note: Typical comonomers are vinyl acetate or methyl acrylate. DuPont created the first acrylic fibers in 1941 and trademarked them under the name Orlon. It was first developed in the mid 1940s but was not produced in large quantities until the 1950s .
