Question
Question: The monomer styrene has a structural formula ________. (A) \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\...
The monomer styrene has a structural formula ________.
(A) C6H5−CH=CH2
(B) C6H5−CH2=CH2
(C) C5H6−CH=CH2
(D) C5H2−CH2=CH2
Solution
The molecule that bonds to two other identical molecules to form a polymer is known as a monomer. The monomer is the repeating unit of the polymer. Most common example of a polymer is polythene(polyethylene), which is a polymer of ethene.
Complete answer:
Styrene is an organic compound and appears as a colourless and oily liquid. Styrene can evaporate easily and has a sweet smell. Styrene occurs in plants and foods like cinnamon, coffee beans, balsam trees and peanuts. It is also found in coal tar. Styrene is flammable in nature. Long exposure to styrene can cause cancer in human beings. Other names of styrene are ethylbenzene, vinylbenzene, phenylethene, phenylethylene, cinnamene, styrol, styrolene and styropor.
The molecular formula for styrene is C8H8.
Styrene has a vinyl group (−CH=CH2) present in it. The presence of vinyl groups allows styrene to polymerize. Thus, styrene is a monomer.
The structure of styrene is as follows:
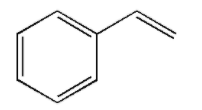
The polymerised products of styrene are polystyrene, styrene-butadiene, rubber, styrene-butadiene latex, etc. all these materials are used in manufacturing rubber, plastic, insulation, etc.
Styrene is prepared industrially by dehydrogenation of ethylbenzene, oxygenation of ethylbenzene, pyrolysis of gasoline, from the reaction of toluene and methanol, from the reaction of benzene and ethane, etc. Laboratory synthesis of styrene is done by decarboxylation of cinnamic acid.
The structural formula for monomer styrene is C6H5−CH=CH2.
**Thus, the correct option is (A) C6H5−CH=CH2.
Note:**
Styrene polymerizes spontaneously and does not need any external initiator. Thus, styrene is an autopolymeriser. Thus, there is a risk of thermal runaway and explosion. Styrene is a ‘known carcinogen’. Styrene affects vision and hearing functions.
