Question
Question: The monomer of natural rubber is: A. butadiene B. chloroprene C. isoprene D. styrene...
The monomer of natural rubber is:
A. butadiene
B. chloroprene
C. isoprene
D. styrene
Solution
The chemical name of natural rubber is polyisoprene. It is used in a variety of applications, ranging from the erasers we use in our stationery, to the tyres of a huge aircraft. Monomer has a five carbon chain.
Complete step by step answer:
-A monomer could be a small molecule that reacts with an analogous molecule to create a bigger molecule. it's the littlest unit in an exceedingly polymer, which is usually a macromolecule with high mass. Monomers are the building blocks for biological macromolecules like DNA, RNA, proteins and carbohydrates.
-There are mainly four types of monomer which includes sugars, amino acids, fatty acids, and nucleotides.
-Polymers are a category of synthetic substances which composes of multiples of simpler units called monomers discussed above. Polymers are chains with an unspecified number of monomeric units. That is polymers are composed of monomers by joining them.
A monomer could be a molecule that forms the fundamental unit for polymers, which are the building blocks of proteins. Monomers bind to other monomers to create repeating chain molecules through a process called polymerization.
-Orlon is created from polymerized acrylonitrile. The acrylic is dissolved in a very solvent, then extruded through spinnerets to supply long, continuous filaments. The smooth, thermoplastic fibers are proof against wrinkles, chemicals, UV light, weathering, insects, mildew, and moisture. Now let's come to the solution. The structure of natural rubber is:
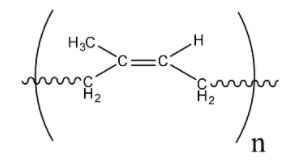
Natural rubber is also known as polyisoprene so it's monomer is isoprene H2C=C(CH3)−CH=CH2
Note: The simplest technique to identify a monomer is to seem at its structure. It always contains different combinations of atoms that together form a singular molecule having a chemical formula in accordance with the overall formula of that class. for instance, the final formula for monomers of carbohydrates is (CH2O)x.
